Meeting Summary
Fixed-ratio combinations, the co-administration of two injectable therapies in a formulation that can be adjusted through titration, are changing the Type 2 diabetes mellitus management paradigm. Current treatment guidelines for glucose control rely heavily on a stepwise approach; however, that can be inconsistently followed and relatively indifferent to the complex pathophysiology of Type 2 diabetes mellitus. Fixed-ratio combinations have targeted actions that complement other treatments. Basal insulin plus a glucagon-like peptide 1 receptor agonist (GLP-1 RA) represent one such combination that offers an efficacious approach to control both fasting and postprandial glucose, key determinants of glycaemic and clinical outcomes.
Two fixed-ratio combinations, insulin glargine 100 U/mL plus lixisenatide (iGlarLixi) and insulin degludec plus liraglutide (IDegLira), are currently available in the European Union (EU) and USA. Clinical evidence from pivotal, Phase III trials with iGlarLixi and IDegLira have demonstrated their robust glycated haemoglobin (HbA1c)-lowering effects, which are associated with mitigation of side effects commonly experienced with the individual components, including basal insulin-related body weight gain and GLP-1-related gastrointestinal adverse events. The spectrum of clinical benefits associated with these titratable fixed-ratio combinations may offer a more compelling case for earlier and effective use of these therapies that better addresses the complex underlying pathophysiology of Type 2 diabetes mellitus.
Symposium Overview
Doctor Elizabeth Seaquist
Diabetes care is evolving. Advances in our understanding of diabetes pathophysiology and treatment now permit individualised therapy based on patient-centred treatment plans that provide the best evidence-based therapies, while minimising personal burden. This personalised approach to treatment requires that therapeutic goals go beyond glycated haemoglobin control to include patient identified outcomes of value such as side effects, cost, and minimal interference with daily living.
Introduction
Doctor Julio Rosenstock
Fixed-dose oral and fixed-ratio injectable combinations of basal insulin plus a glucagon-like peptide 1 receptor agonist are changing the Type 2 diabetes mellitus management paradigm. Conceptually, a fixed-dose or fixed-ratio combination should exhibit the following characteristics:
- Components should exhibit complementary actions.
- Glycaemic control should be better than with each individual component.
- Combined doses may be lower than each individual component alone.
- Side effects should not be increased and ideally be mitigated.
- Treatment is simplified and may improve adherence and persistence.
- Cost should be lower than the sum of the costs of each individual component.
Fixed-dose combinations represent the co-administration of two oral antihyperglycaemic therapies in the same tablet formulation. Numerous fixed-dose combinations are currently available, including metformin plus sodium-glucose cotransporter 2 (SGLT2) inhibitor formulations. However, the fixed-dose nature of these combinations means that adjustments are limited by tablet options. Fixed-ratio combinations represent the co-administration of two injectable therapies in the same injection, which can be adjusted through titration. These formulations offer the ability to titrate doses in accordance with the individual response and tolerance.
Two approved fixed-ratio combinations, iGlarLixi and IDegLira, are currently available. In Europe, iGlarLixi and IDegLira are indicated for adults with Type 2 diabetes mellitus inadequately controlled with oral antidiabetic drugs (OAD),1,2 while in the USA, iGlarLixi and IDegLira are indicated for adults with Type 2 diabetes mellitus inadequately controlled with basal insulin.3,4 Regulatory approval of iGlarLixi and IDegLira in the OAD-failure population was supported by evidence from the pivotal Phase III trials LixiLan-O and DUAL I,5,6 respectively, while approval in the basal insulin-failure population was supported by evidence from the LixiLan-L and DUAL II trials,7,8 respectively.
The design of the LixiLan and DUAL programmes was relatively similar, with the exception of a few key differences; the LixiLan trial contained a titration lead-in period, whereas the DUAL trials did not, and fasting plasma glucose targets were modestly higher in the LixiLan versus DUAL trials.5-8 Data from the LixiLan-O and DUAL I trials revealed study populations with generally comparable characteristics, with the exception that the LixiLan-O population was slightly older and had diabetes for longer.5,6 Importantly, the studies demonstrated robust and comparable HbA1c-lowering with both iGlarLixi and IDegLira (final HbA1c: 6.5% and 6.4%, respectively). Similarly, LixiLan-L and DUAL II enrolled comparable patient populations and, despite differences in study design (i.e. the lack of a titration lead-in period in DUAL II), demonstrated robust and comparable reductions in HbA1c (final HbA1c: 6.9% in both trials).7,8 Taken together, evidence from the LixiLan and DUAL programmes demonstrated that fixed-ratio co-formulations of a basal insulin and GLP-1 RA provide robust glycaemic control with a favourable safety profile, by mitigating side effects of each component, and with no increased regimen complexity.
A Matter of Urgency: Simultaneous Intensification with Fixed Ratio Combinations
Doctor James R. Gavin III
Key Points
- The sequential approach to Type 2 diabetes mellitus management is compounded by substantial clinical inertia at each intensification step.
- Many individuals do not achieve their glycaemic targets despite optimised treatment with multiple OAD and/or injectable therapies.
- Persistent postprandial hyperglycaemia, a predictor of cardiovascular mortality, represents an unmet need in the management of patients with Type 2 diabetes mellitus.
- Fixed-ratio combinations of a basal insulin plus GLP-1 RA provide a means to target both fasting and postprandial glucose levels.
- Basal insulin/GLP-1 RA combinations offer significant clinical benefits to patients, including improved glycaemic control and mitigation of the side effects commonly experienced with the individual components.
- Compared with the sequential approach, simultaneous intensification with a fixed-ratio combination in patients failing oral therapy may serve to maximise the benefits of the fixed-ratio combination and mitigate the risk of clinical inertia.
There is an urgent need for simultaneous treatment intensification in patients with Type 2 diabetes mellitus. Many individuals do not achieve their glycaemic targets despite optimised treatment with multiple OAD and/or insulin or other injectable therapies.9 Evidence from the European PANORAMA study, a cross-sectional analysis of glycaemic control data collected in 5,817 patients with Type 2 diabetes mellitus aged ≥40 years, showed that up to ~40% of patients were not at HbA1c target (≤7.0%).9
Furthermore, the proportion of patients who achieved an HbA1c ≤7.0% decreased with increasing treatment complexity (76.1% in patients receiving one OAD versus 36.1% in patients receiving injectable therapy). This lack of glycaemic control is due, in part, to the sequential treatment approach, which is compounded by substantial clinical inertia at each intensification step. Clinical data indicate that the median time from OAD treatment initiation to the addition of a second OAD is 1.6–2.9 years, while the time to addition of a third OAD is 6.9–7.2 years. A further 6.0–7.1-year delay exists before intensification with insulin.10 A persistent problem and unmet need in Type 2 diabetes mellitus treatment is how best to manage the postprandial hyperglycaemia that precedes fasting hyperglycaemia during disease progression. Both basal and postprandial elevations contribute to the hyperglycaemic exposure of diabetes. However, current therapies are predominantly effective in controlling the basal component.11 Findings from prospective, cohort studies show that postprandial hyperglycaemia is an independent predictor of mortality outcomes in patients with pre and overt diabetes,12,13 while an abundance of studies have reported a link between postprandial blood glucose levels and cardiovascular mortality.12-15
Fixed-ratio combinations could potentially offer a number of benefits to patients. These include earlier achievement and greater persistence of glycaemic and other therapeutic goals, and a potential reduction in the risk of side effects with lower doses of the combined drugs versus uptitration of single doses. Fixed-ratio combinations may help to address the multiple physiologic defects associated with Type 2 diabetes mellitus. Furthermore, these formulations may potentially delay underlying disease progression and disease-related vascular complications. The mode of action of basal insulins, to predominantly provide fasting glucose control, is complementary to the postprandial glucose control provided by GLP-1 RA. Together, these anti-hyperglycaemic agents offer a complementary approach to glycaemic control that may help address the postprandial element of hyperglycaemia that persistently limits current diabetes therapies.16,17 Two basal insulin/GLP-1 RA fixed-ratio combinations have been approved to date: iGlarLixi and IDegLira.
Evidence for Fixed-Ratio Basal Insulin/ Glucagon-like Peptide 1 Receptor Agonist Combination Therapy
The efficacy and safety of iGlarLixi was established in the LixiLan-O trial, which demonstrated that iGlarLixi was associated with a superior HbA1c reduction compared with its individual components, insulin glargine 100 U/mL and lixisenatide (1.6% versus 1.3% and 0.9%, respectively; p<0.0001).5 In addition, iGlarLixi was associated with greater reductions in 2-hour postprandial glucose levels compared with insulin glargine 100 U/mL or lixisenatide alone.5 A significantly greater proportion of patients achieved an HbA1c target <7% with iGlarLixi than with insulin glargine 100 U/mL and lixisenatide (74% versus 59% and 33%, respectively; p<0.0001).5 Modest weight loss was observed with the iGlarLixi arm (0.3 kg), compared with weight gain (1.1 kg) in the insulin glargine 100 U/mL arm, while the incidence of hypoglycaemia was comparable between both arms.5 In patients with a baseline HbA1c of ≤9% and those who were receiving ≥2 OAD at baseline, iGlarLixi was associated with robust and significantly superior HbA1c-lowering (p≤0.03), and a greater proportion of patients achieving glycaemic goal (HbA1c <7%), compared with its individual components.18,19
Together, these data indicate that patients with advanced disease, including those in whom an injectable therapy may be considered, are well suited to the effects of iGlarLixi. In terms of safety and tolerability, the slow titration of the fixed-ratio combination resulted in fewer gastrointestinal (GI) events and a reduced incidence of discontinuation due to GI events with iGlarLixi versus insulin glargine 100 U/mL and lixisenatide given as monocomponents.5 Evaluation of IDegLira in the DUAL I trial produced comparable results to those observed with iGlarLixi. Analyses of DUAL I showed that IDegLira was associated with superior HbA1c reduction compared with insulin degludec or liraglutide alone (1.9% versus 1.4% and 1.3%, respectively; p<0.0001).6 In addition, a significantly greater proportion of patients achieved an HbA1c <7% with IDegLira than its individual components (p<0.0001).6 In terms of body weight change, IDegLira treatment was associated with weight loss (0.5 kg) versus weight gain in the insulin degludec arm (1.6 kg).6
Evaluation of iGlarLixi in LixiLan-L showed iGlarLixi versus insulin glargine 100 U/mL was associated with superior HbA1c reduction (1.1% versus 0.6%; p<0.0001), a robust reduction in 2-hour postprandial glucose and fasting plasma glucose levels, a higher proportion of patients at HbA1c targets (p<0.0001), weight loss, and no increased risk of hypoglycaemia.7 Similarly in DUAL II, IDegLira compared with insulin degludec was associated with superior HbA1c-lowering, a greater proportion of patients at HbA1c targets, and weight loss.8
Taken together, these data highlight the numerous clinical benefits that fixed-ratio combinations of a basal insulin and a GLP-1 RA can potentially offer, including simultaneous targeting of both fasting plasma glucose and postprandial glucose, substantial reductions in the side effects of the mono-components, and no increase in the risk of hypoglycaemia compared with basal insulin. For patients who are struggling to manage their disease, there is an urgent need for earlier, intensive glycaemic control. Indeed, post-hoc analyses of 4,119 patients from the ACCORD20 study demonstrated that early glycaemic goal attainment is predictive of persistent and improved glycaemic control.
Conversely, a global study of 40,627 patients with Type 2 diabetes mellitus initiating basal insulin showed that failure to achieve HbA1c ≤7% at 3 months post initiation was associated with an increased risk of failing to achieve this target at 24 months (odds ratio: 3.70; 95% confidence interval [CI]: 3.41–4.00).21 In the LixiLan-O trial, patients treated with iGlarLixi were more likely to achieve early HbA1c control; time to target HbA1c <7% was 85 days with iGlarLixi, 166 days with insulin glargine 100 U/mL, and 218 days with lixisenatide.22
In summary, fixed-ratio combinations offer significant clinical benefits. Compared with sequential intensification, simultaneous intensification with a fixed-ratio combination in patients failing oral therapy may serve to maximise the benefits of the fixed-ratio combination and mitigate the risk of clinical inertia. iGlarLixi is also associated with earlier achievement of HbA1c targets, a predictor of long-term control.
Who Can Benefit and How to Use: Assessing Clinical Utility
Doctor Neil Skolnik and Ms Lucia Novak
Key Points
- Fixed-ratio combinations offer a simple and efficacious therapeutic approach for advancing therapy in patients with Type 2 diabetes mellitus
- Practical considerations when initiating a patient on a fixed-ratio combination include providing education on the causes and duration of potential side effects, as well as how to minimise the likelihood of these events occurring
- Such practical support will help patients to successfully initiate and persist with their fixed-ratio combination treatment
The body of clinical evidence that supports the efficacy and safety of fixed-ratio combinations indicates that they offer an efficacious therapeutic approach that may reduce treatment complexity.5-8,10,23
How to Initiate Fixed-Ratio Combinations
iGlarLixi is available in two dose pens, the 10–40 U pen and the 30–60 U pen, which deliver 10–40 U insulin glargine 100 U/mL plus 5–20 µg/day lixisenatide and 30–60 U insulin glargine 100 U/mL plus 10–20 µg/day lixisenatide, respectively.1,3 IDegLira is supplied in a single, multiple-dose pen with a maximum permitted dose of 50 U insulin degludec and 1.8 mg liraglutide.2,4 The starting dose of iGlarLixi or IDegLira is dependent upon the patient’s basal insulin experience. For iGlarLixi, patients who are insulin-naïve or receiving basal insulin therapy at a dose of <30 U/day should start treatment on the 10–40 U pen. Patients who are basal insulin experienced and receiving >30 U/day should start treatment on the 30–60 U pen.1,3 For IDegLira, insulin-naïve patients would initiate treatment at a dose of 10 U, and basal insulin experienced patients (irrespective of their existing insulin dose) should initiate treatment at a dose of 16 U.2,4 Dosing of iGlarLixi and IDegLira is conducted in accordance with the patient’s basal insulin needs. Titration of iGlarLixi and IDegLira doses is based on fasting self-monitored plasma glucose levels and slow uptitration. This slow titration means that increases in the corresponding dose of the GLP-1 RA component are gradual and relatively small.
Patient Education: Practical Considerations
To ensure successful initiation and persistence with fixed-ratio combination therapies, it is important to discuss treatment expectations with patients before beginning treatment to provide them with the tools and understanding they require in order to overcome any potential barriers that they may experience with these medications. Common complications associated with GLP-1 RA treatment are the occurrence of GI events, specifically nausea and vomiting.5-8 The increased satiety and slowed gastric emptying produced by these agents may lead to an increased sense of fullness that some patients who are new to GLP-1 RA are not accustomed to experiencing. As a result, this could potentially lead to premature treatment discontinuation.24 In light of this, it is important to highlight to patients that the nausea associated with fixed-ratio combination therapy is usually transient in nature. It is also important to highlight the benefits of fixed-ratio combinations, such as the mitigation of weight gain that can be associated with basal insulin therapy.
Another approach to help patients initiate and persist with their fixed-ratio combination therapy could be to suggest changes to their behaviour.24 For example, the risk of experiencing GI events can be minimised through slow titration of the dose. Meals containing a high fat content may slow gastric emptying further and contribute to the worsening of nausea symptoms. Therefore, reducing fat intake, mindful eating (i.e. eating slowly and stopping eating when fullness is first sensed), and decreasing portion sizes may also help to reduce the likelihood of experiencing nausea.
Take Home Messages
Fixed-ratio combinations offer a simple and effective therapeutic approach for advancing therapy in patients with Type 2 diabetes mellitus. Efforts to provide patients with supporting education will help patients to successfully initiate and persist with these therapies.
Reflections on Injectables for Type 2 Diabetes Mellitus: Let’s Move On!
Doctor Julio Rosenstock
Key Points
- Current clinical guidelines advocate the use of injectable glucose-lowering agents when metformin alone, or in combination with additional OAD, fails to achieve individualised glycaemic goals.
- Injectable therapies, including basal insulin and GLP-1 RA, are well established options for the sequential intensification of metformin treatment in patients unable to achieve glycaemic control.
- However, the effectiveness of traditional, injectable therapies may be hindered by factors such as the fear of weight gain or hypoglycaemia with basal insulin therapy, or concerns regarding treatment complexity.
- The recent introduction of the titratable fixed-ratio combinations iGlarLixi and IDegLira signifies the beginning of a new era for injectable therapies in Type 2 diabetes mellitus.
- Titratable fixed-ratio formulations offer a unique therapeutic approach that can deliver the benefits of both a basal inulin and a GLP-1 RA simultaneously, while mitigating basal insulin-related body weight gain and GLP-1-related GI adverse events, and may potentially replace sequential injectable regimens in the Type 2 diabetes mellitus treatment paradigm.
For many years, the Type 2 diabetes mellitus treatment paradigm has followed a common sequential principle: initiate treatment with metformin and then progressively add therapies according to increasing HbA1c. Indeed, the American Diabetes Association/European Association for the Study of Diabetes joint guidelines advocate metformin as a first-line agent, followed by the potential addition of further oral agents and eventually basal insulin or a GLP-1 RA as a dual or triple therapy, before moving sequentially to basal-bolus insulin.25 In addition to initial metformin monotherapy, the American Diabetes Association guidelines also suggest using dual therapy as a first-line treatment in patients with HbA1c ≥9%, before sequentially moving to triple therapy and then combination injectable therapy.26 The American Association of Clinical Endocrinologists guidelines advocate a more aggressive approach, in which dual therapy is recommended as a first-line treatment if HbA1c is ≥7.5%, while monotherapy with either metformin, GLP-1 RA, or SGLT2 is recommended if HbA1c <7.5%.27 However, the sequential therapeutic approach, as recommended by most clinical guidelines, has failed, mainly due to clinical inertia. Looking forward, it is likely that initial dual therapy will become the standard of care. Furthermore, it is conceivable that initial therapy with a combination of metformin and an SGLT2 inhibitor could become the standard treatment for newly diagnosed Type 2 diabetes mellitus, given the emerging evidence supporting a cardiovascular benefit of the SGLT2 inhibitor class.28,29
Choosing a First Injectable in Oral Antidiabetic Drug Failure
In order to select the most appropriate first injectable for patients who are not responding to OAD, it is important to consider key learnings generated from the body of clinical evidence available for basal insulins and GLP-1 RA. Single and pooled analyses of treat-to-target trials with insulin glargine 100 U/mL demonstrate the consistent and robust HbA1c-lowering effect of this therapeutic approach.30-33 However, these effects are only achievable through the implementation of consistent and systematic basal insulin titration not often followed in clinical practice. Additional limitations of basal insulin therapy include a low, but persistently elevated, increase in the risk of hypoglycaemia, potential weight gain of ~1–3 kg, and low adherence to titration.30-33 Analyses from a USA retrospective study conducted in 274,102 patients with Type 2 diabetes mellitus also demonstrated that up to 50–60% of patients receiving insulin glargine 100 U/mL discontinued their therapy after 12 months, irrespective of whether they were insulin experienced or new to insulin therapy (<12 months). Neutral protamine Hagedorn insulin was associated with the lowest rates of treatment persistence.34
Head-to-head trials among GLP-1 RA have demonstrated the robust glycaemic efficacy of this class, with HbA1c reductions ranging from approximately 0.6–1.9%.35-40 These studies have also demonstrated substantial body weight reduction associated with GLP-1 RA therapy (ranging from 2.0–2.5 kg).35-38 However, adherence and persistence with GLP-1 RA is limited by the adverse event profile associated with these therapies. GI adverse events are common with GLP-1 RA, with 18–35% of patients experiencing nausea, 8–12% experiencing vomiting, and 9–14% experiencing diarrhoea. Although nausea and vomiting generally subside in the weeks to months following treatment initiation, a large proportion of patients will discontinue their GLP-1 RA treatment during this preliminary period due to a lack of understanding that these events are transient in nature and the lack of a support system in clinical practice, which is available in randomised clinical trials. Treatment persistence with dulaglutide, exenatide, and liraglutide was evaluated in a 6-month observational study in patients with Type 2 diabetes mellitus (N=2,470), in which discontinuation was defined as either a 45-day or 60-day gap from the index date of GLP-1 RA initiation to the final day’s supply from the last claim of the GLP-1 RA treatment.39 These analyses showed that higher proportions of patients met the criteria for early discontinuation (no claim for the specified GLP-1 RA within the 45-day gap) compared with delayed discontinuation. Overall, 31–53% of patients discontinued early versus 26–48% who had delayed discontinuation with this trend being present for all three GLP-1 RA evaluated in the study.39
Advancing Basal Insulin and Glucagon-like Peptide 1 Receptor Agonists
Guideline recommendations for the sequential intensification of basal insulin permits the addition of prandial insulin (i.e. basal-bolus) or a GLP-1 RA.25-27 A recent head-to-head study evaluated these prandial treatment options in patients with Type 2 diabetes mellitus inadequately controlled on basal insulin glargine with or without additional OAD. Analyses demonstrated that lixisenatide once daily was associated with similar HbA1c reductions versus insulin glulisine once daily and fairly comparable with thrice daily, when added to insulin glargine as a basal/bolus (final HbA1c: 7.2%, 7.2%, and 7.0%, respectively).40 Similar findings were demonstrated by a 30-week, randomised, non-inferiority study, which evaluated the addition of a GLP-1 RA (exenatide twice weekly) versus meal-time insulin (lispro three times daily) in patients with Type 2 diabetes mellitus inadequately controlled on basal insulin glargine plus metformin (N=510).41 At study end, reductions in HbA1c were similar between the exenatide twice weekly and insulin lispro treatment arms (1.1% in both arms), with a final HbA1c of 7.2%.41 In addition, exenatide twice weekly was associated with substantial body weight reduction (2.5 kg), while meal-time insulin lispro was associated with body weight gain (2.1 kg). Interestingly, evidence from a series of subsequent studies have shown significant reductions in HbA1c and body weight with once-weekly GLP-1 RA; these include once-weekly albiglutide, exenatide, and dulaglutide, which have shown final HbA1c values of 7.7%, 7.6%, and 6.9%, respectively.41-43
Simultaneous intensification with a fixed-ratio combination of basal insulin plus a GLP-1 RA offers an alternative therapeutic option to prandial interventions. Clinical evidence from the LixiLan and DUAL clinical programmes with iGlarLixi and IDegLira, respectively, demonstrated that these titratable fixed-ratio combinations produce robust HbA1c-lowering, mitigation of basal insulin-related body weight gain, and mitigation of GLP-1-related GI adverse events.5-8
Exploring Sequential Versus Simultaneous Addition of Glucagon-like Peptide 1 Receptor Agonist to Basal Insulin
The differences in efficacy of titratable fixed-ratio combinations and sequential intensification of treatment in accordance with treatment guidelines has been questioned. However, no head-to-head studies comparing these two treatment regimens have yet been carried out. Therefore, propensity score matching was used as a hypothesis-generating, exploratory exercise to investigate if titratable fixed-ratio combinations offer a treatment benefit over correctly executed simultaneous intensification.44,45 If sequential intensification was performed systematically, and in accordance with guideline recommendations, it is conceivable that outcomes could be improved. Therefore, propensity score matching was used to indirectly compare simultaneous administration of iGlarLixi in the LixiLan-O trial (n=469) with sequential therapy, starting with initial insulin glargine 100 U/mL therapy for 12 weeks, followed by addition of lixisenatide in the GetGoal Duo-1 trial in patients with Type 2 diabetes mellitus who had failed oral therapy but remained on metformin (n=223).44 At Week 24, data from 87 matched pairs revealed that iGlarLixi was associated with a significantly greater reduction in HbA1c compared with sequential administration of insulin glargine 100 U/mL and then lixisenatide (final HbA1c 6.4% versus 7.0%, respectively; p<0.0001) in patients with Type 2 diabetes mellitus uncontrolled with OAD (Figure 1).44 Furthermore, a greater proportion of patients achieved the HbA1c target (<7%) with iGlarLixi versus sequential intensification (79% versus 51%; p<0.0001) (Figure 1).44
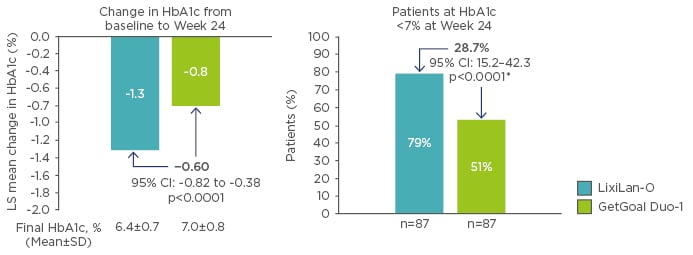
Figure 1: HbA1c changes in propensity scored-matched populations.44
*Weighted average of proportion difference between treatment groups from each strata (randomisation strata of HbA1c [<8.0, ≥8.0%]) using Cochran-Mantel-Haenszel weights.
CI: confidence interval; HbA1c: glycated haemoglobin; LS: least squares; SD: standard deviation.
© 2017 by the American Diabetes Association®; Diabetes June 2017,Volume 66;Suppl 1. Reprinted with permission from the American Diabetes Association®.
Similar results were generated from a propensity score matching analysis of data from the LixiLan-L (n=367) and GetGoal Duo-2 (n=298) trials in patients with Type 2 diabetes mellitus who had failed basal insulin therapy with or without metformin.45 At study end, data from 241 matched pairs revealed that iGlarLixi was associated with a significantly greater reduction in HbA1c compared with sequential administration of insulin glargine 100 U/mL and lixisenatide (final HbA1c: 6.8% versus 7.3%, respectively; p<0.0001).45 In addition, significantly more patients achieved the HbA1c target (<7%) with iGlarLixi than sequential intensification (62% versus 33%; p<0.0001). These hypothesis-generating, exploratory data are particularly compelling given that this particular patient population is often difficult to treat due to the advanced state of their disease.
Take Home Messages
There is a growing body of clinical evidence supporting the clinical benefits of simultaneous intensification of therapy with a fixed-ratio combination of basal insulin plus a GLP-1 RA in patients with Type 2 diabetes mellitus. Importantly, these titratable fixed-ratio combinations have the potential to ultimately replace the standard, sequential, injectable regimens that, slowly but surely, will become a thing of the past in the management of Type 2 diabetes mellitus.
Conclusions
Titratable fixed-ratio combinations of basal insulin and a GLP-1 RA are changing the Type 2 diabetes mellitus management paradigm. Current guidelines advocate a sequential approach to treatment. However, an abundance of clinical evidence indicates that significant clinical inertia exists at each intensification step. As a result, many patients fail to achieve their personalised glycaemic goals despite optimised treatment with OAD with or without injectable therapy. Fixed-ratio combinations of basal insulin plus a GLP-1 RA represent an efficacious approach to control both fasting and postprandial glucose, key determinants of glycaemic and clinical outcomes. Clinical evidence from pivotal Phase III trials with the two currently available fixed-ratio combinations, iGlarLixi and IDegLira, have demonstrated their robust HbA1c-lowering effects. Furthermore, the titratable nature of these new formulations according to clinical response and tolerance enables patients to reach levels of glycaemic control with mitigated side effects that are unprecedented in the management of Type 2 diabetes mellitus.








