Abstract
Diabetic dyslipidaemia (DD) comprises a complex group of potentially atherogenic lipid and lipoprotein abnormalities, including both quantitative and qualitative changes. It is characterised by low high-density lipoprotein cholesterol, elevated low-density lipoprotein cholesterol (LDL-C), and a higher prevalence of small, dense LDL particles, as well as elevated fasting and postprandial triglycerides. Patients with Type 2 diabetes mellitus have an increased prevalence of lipid abnormalities and controlling dyslipidaemia in these patients has a big impact on morbidity and mortality. Lifestyle changes are still the pillar of treatment for DD and statins are the drugs of choice that decrease LDL-C and reduce cardiovascular events and cardiovascular death, either in primary or secondary prevention, in diabetic patients. Pitavastatin has a number of pleiotropic effects that reduce the metabolic changes associated with adiposity and improve glucose metabolism, which distinguishes it from other statins. New treatments, such as PCSK9 inhibitors, have proven to be powerful LDL-C-lowering agents; however, the need for long-term safety studies and the high associated costs are the main challenges. Future treatments, such as an intracellular PCSK9 inhibitor, a dual proliferator-activated receptor-alpha/gamma agonist, and bempedoic acid, are in development. The aim of this article is to review the pathophysiology of DD and discuss its role in cardiovascular event risk and treatment, as well as to study the effects of lipid-lowering therapy on glucose metabolism and the outcomes of antidiabetic treatment on dyslipidaemia.
INTRODUCTION
Diabetic dyslipidaemia (DD) is characterised by low levels of high-density lipoprotein cholesterol (HDL-C), elevated levels of low-density lipoprotein cholesterol (LDL-C), and a higher prevalence of small, dense LDL particles, as well as elevated fasting and postprandial triglycerides (TG). The major link between diabetes and the higher risk of cardiovascular disease in these patients is related to these common lipid abnormalities.1 However, the underlying pathophysiology is only partially understood. Alterations in insulin-sensitive pathways, increased concentrations of free fatty acids (FFA), and low-grade inflammation play a role and result in the overproduction and decreased catabolism of TG-rich lipoproteins (TRL) of intestinal and hepatic origin.2 This article will review the pathophysiology of DD, discuss its role in cardiovascular event risk and treatment, and evaluate the effects of hypolipidaemic treatment on glucose metabolism and the outcomes of antidiabetic treatment in dyslipidaemia. The future directions and perspectives of non-statin therapies are also reviewed.
PATHOPHYSIOLOGY AND LIPID ABNORMALITIES IN TYPE 2 DIABETES MELLITUS PATIENTS
DD forms a complex group of potentially atherogenic lipid and lipoprotein abnormalities, including both quantitative and qualitative changes. The main alterations associated with DD (known as the triad of DD) are detailed below.
Increased Plasma Triglycerides
The metabolism of lipids in diabetes, particularly Type 2 diabetes mellitus (T2DM), is influenced by a series of factors, including the degree of glycaemic control and the presence of insulin resistance, which are the most prominent elements. Insulin resistance is the basis of the pathophysiological mechanisms of DD and is closely related to hypertriglyceridaemia and postprandial lipaemia.3 Insulin reduces very LDL (VLDL) production by decreasing circulating levels of FFA, which are substrates of VLDL, and by exerting a direct inhibitory effect on VLDL production in hepatocytes. It has been shown that insulin inhibits the maturation phase of VLDL assembly through the phosphatidylinositol 3-kinase pathway, preventing the transfer of bulk lipids to VLDL precursors. This mechanism is involved in the inhibitory effect of insulin related to the secretion of VLDL. The binding of insulin to its receptor induces tyrosine phosphorylation of insulin receptor substrates, leading to the activation of phosphatidylinositol 3-kinase, which, once activated, induces the transformation of phosphatidylinositol 4,5-bisphosphate into phosphatidylinositol 3,4,5-triphosphate and leads to the activation of Akt, a serine/threonine kinase that is an effector of the metabolic actions of insulin.1
An important consequence of insulin resistance, with respect to lipid metabolism, is the loss of the suppressive effect of insulin on the mobilisation of adipose tissue fat.4 As a result, there is an increase in FFA due to a reduction in the suppression of lipolysis. The lack of suppression of FFA in the postprandial period results as a consequence of the decrease in lipoprotein lipase activity, and the increase in plasma FFA is due to an increase in lipolysis in adipocytes. These form the key mechanisms that underlie the increase in hepatic TG secretion of VLDL.5
In healthy individuals, insulin inhibits the assembly and secretion of VLDL particles through an increase in the degradation of apolipoprotein B (ApoB) and a decrease in the expression of microsomal transfer protein in the hepatocytes. As a consequence, insulin inhibits hepatic secretion of TG-VLDL and ApoB-100.6 In T2DM patients and those with other states of insulin resistance, an increase in microsomal transfer protein expression occurs in the liver, along with an increase in lipid bioavailability (i.e., the flow of FFA), and this leads to an overproduction of TG-VLDL and VLDL-ApoB. In this context, the overproduction of hepatic VLDL corresponds to large, floating VLDL particles, which are a predominant feature of DD. Most of the increase in TRL observed in DD is due to VLDL particles.6
Low Concentrations of High-Density Lipoprotein Cholesterol
The increase in plasma TG presents a central lipid exchange between TRL and HDL particles. There is an increase in the transfer of esterified cholesterol to the TRL, facilitated by the cholesterol ester transfer protein, and the transfer of TG to the HDL particles, which leads to an enrichment of TG in these particles. HDL TG are a suitable substrate for hepatic lipase and hydrolysis produces smaller HDL particles and free ApoA-I that are excreted by the kidneys. The catabolism of small HDL is faster than that of normal HDL, and this results in a reduction in the amount of circulating HDL particles.7
Predominance of Small, Dense Low-Density Lipoproteins and Excessive Postprandial Lipaemia
Small dense LDL particles (Phenotype B) are a prominent feature of DD and the number of these atherogenic particles is increased. It has been repeatedly confirmed that the concentration of plasma TG is the most important determinant of the size of LDL.8 On the other hand, the size of LDL decrease progressively as glucose tolerance worsens, until overt diabetes is achieved. This decrease is greater in women than in men.8
TG of VLDL are the main predictors of LDL size in individuals with T2DM, and kinetic data indicate that VLDL are the precursors of small, dense LDL particles.9 In fact, the long residence time of VLDL in the plasma, due to the reduction of lipolysis, is a prerequisite for the formation of small, dense LDL since it favours the excess lipid exchange of TG and cholesterol esters between TRL and LDL. When the LDL have depleted cholesterol esters and an enrichment of TG, an increase in the action of hepatic lipase results in the formation of the subclass of small, dense particles. Since each particle of LDL contains a molecule of ApoB-100, the number of small, dense LDL is increased and, similarly, the concentration of ApoB-100 increases in direct relation. Consequently, ApoB concentrations are a marker of the number of atherogenic particles and hypertriglyceridaemia with hyper-ApoB-100 is a well-known feature of DD and other conditions.9
CARDIOVASCULAR RISK AND DIABETIC DYSLIPIDAEMIA
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in patients with diabetes.10 Diabetes itself is a major cardiovascular risk factor and the coexistence of other risk factors, such as hypertension and dyslipidaemia, is frequent. In particular, patients with T2DM have an increased prevalence of lipid abnormalities.10 These lipid abnormalities are largely shared between two conditions when defined by HbA1c levels (HbA1c: >6.5% [48 mmol/mol] are classified as T2DM; HbA1c: 5.7–6.4% [39–46 mmol/mol] are classified as prediabetes). Prediabetes is characterised by lower ApoA-1 and HDL cholesterol levels and higher TG levels and ApoB/ApoA-1 ratio. Subjects with diabetes show lower ApoA-1, HDL cholesterol, and TG levels. When subjects treated with lipid-lowering drugs were excluded, no differences in LDL or non-HDL cholesterol were found between subjects with prediabetes and diabetic patients.11
It is very important to address multiple cardiovascular risk factors simultaneously. Thus, the maximum benefit is achieved when diabetes, hypertension, dyslipidaemia, smoking, and albuminuria are considered altogether. Controlling cardiovascular risk factors has a large impact on morbidity and mortality, which has been documented in many clinical trials.10 Statin therapy reduces cardiovascular events and cardiovascular death, either in primary or secondary prevention, in diabetic patients.10-15 Data from meta-analyses (>18,000 patients; mean follow-up: 4.3 years) show a 9% reduction in all-cause mortality and 13% reduction in vascular mortality for each 39 mg/dL reduction in LDL-C.16
Statins are the drug of choice for LDL-C treatment. Nowadays, the initiation of a statin in diabetic patients is mainly guided by focussing on the patient, particularly considering their age and history of previous cardiovascular events (primary or secondary prevention). As in non-diabetic people, absolute risk reduction will be greatest in subjects with a higher risk, but the benefits of statin therapy in people with diabetes at a moderate or low risk for ASCVD are also documented.17
Estimating cardiovascular risk in diabetic people can be challenging and diabetes itself confers an increased risk of ASCVD. Risk calculators are not useful in this population because they often do not include diabetes, its duration, or the presence of complications. Thus, the management of lipids in these patients relies on published evidence, guideline indications, and clinical judgement.
At diagnosis, or in those with a short duration of disease, diabetes is not a coronary artery disease (CAD)-risk equivalent state.18,19 In general, risk levels approach CAD-risk equivalence after around a decade or in those with proteinuria or a low estimated glomerular filtration rate.20,21 Emerging data suggest that patients who develop T2DM at a younger age have a high complication burden.22 People with diabetes and CAD have a vascular risk that exceeds that of CAD patients without diabetes, and have a substantially lower life expectancy.23
TREATMENT OF DIABETIC DYSLIPIDAEMIA
Treatment Targets
The American Diabetes Association (ADA) guidelines10,24 recommend the following treatment for DD:
1. People <40 years:
- No ASCVD: no statin.
- With ASCVD: high-intensity statin (Table 1).
2. People ≥40 years:
- No ASCVD: moderate-intensity statin (Table 1).
- With ASCVD: high-intensity statin (Table 1) .
With ASCVD, if LDL-C is ≥70 mg/dL, despite a maximally tolerated statin dose, consider an additional LDL-lowering therapy (e.g., ezetimibe, PCSK9 inhibitor).
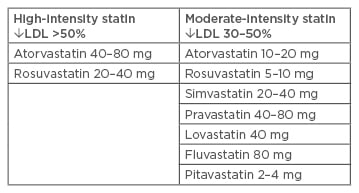
Table 1: High and moderate-intensity statin therapies.
LDL: low-density lipoprotein.
Adapted from American Diabetes Association24
The Joint European Society of Cardiology (ESC) guidelines18 also recommend lipid-lowering agents (principally statins) in patients with diabetes (Type 1 or Type 2) >40 years of age. They use LDL targets to guide therapy:
- In patients with diabetes at a very high risk (diabetes with target organ damage such as proteinuria or with a major risk factor, such as smoking or marked hypercholesterolaemia or marked hypertension), the LDL-C target is <1.8 mmol/L (<70 mg/dL) or a reduction of at least 50% if the baseline LDL-C is between 1.8 and 3.5 mmol/L (70 and 135 mg/dL).
- In patients with diabetes at a high-risk (most other people with diabetes, with the exception of young people with Type 1 diabetes mellitus and without major risk factors that may be at low or moderate risk), the LDL-C target is <2.6 mmol/L (<100 mg/dL) or a reduction of at least 50% if the baseline LDL-C is between 2.6 and 5.1 mmol/L (100 and 200 mg/dL).
ESC guidelines also highlight that non-HDL-C is a reasonable and practical alternative target because it does not require fasting. Non-HDL-C secondary targets include:
- 2.6 mmol/L (100 mg/dL) for very high-risk subjects.
- 3.3 mmol/L (130 mg/dL) for high-risk subjects.
There is increasing evidence of a very high relative risk in younger individuals with T2DM so additional studies and guidance are needed in this group.22
Non-Pharmacological Therapy
Lifestyle Changes
Lifestyle changes remain the pillar of treatment, not only for DD but for diabetes in general, and are strongly advised for diabetic patients.24 Nutritional therapy should be adapted to each patient and there is not enough evidence to recommend an ideal distribution of principal macronutrients. In terms of cardiovascular risk, the total amount of fat consumed is less important than the type of fat. In general, recommendations must focus on reducing cholesterol and saturated and trans-fat intake, and increasing fibre, n-3 fatty acids, and plant stanols/sterols intake. A Mediterranean-style diet (rich in monounsaturated and polyunsaturated fats) has been shown to be effective at improving lipid profile and glycaemic control.24 In addition, the loss and maintenance of weight (of at least 5%), if indicated, is associated with improvements in lipid levels.10,25 Dietary fats and processed foods are rich in advanced glycation end-products, which are primarily elevated in diabetic patients, and an excessive consumption of these can increase the total pool in the body. Advanced glycation end-products can raise oxidative stress and increase arterial endothelial dysfunction.26
Physical activity, fundamentally aerobic exercise alone and in combination with resistance exercise, improves certain cardiovascular risk factors, including blood pressure, glucose metabolism, BMI, and waist circumference, but the effects on lipid parameters are inconsistent and sometimes contrasting. Combined exercise has shown better outcomes than each exercise separately.27 The ADA recommends ≥150 minutes of moderate-to-vigorous aerobic activity weekly, divided between at least 3 days, combined with two or three sessions per week of resistance exercise on non-consecutive days. A combination of exercise and diet obtains better weight loss than diet alone and shows better improvements in lipid profile than exercise alone.25,27 Smoking cessation is associated with elevation of HDL-C levels,28 but there are no specific data in diabetic patients. Alcohol consumption must be moderate and should be avoided in hypertriglyceridaemia patients.10
Pharmacological Therapy
Classic Drugs
Diabetes is associated with a marked increased risk of premature ASCVD. Patients with diabetes have a high prevalence of lipid abnormalities and there is strong evidence that lowering cholesterol blood levels improves cardiovascular outcomes, even in patients with unremarkable lipid profiles.29-31
Statins are the first-choice drugs for lowering LDL-C in patients with diabetes and work by inhibiting HMG-CoA reductase, which is responsible for the production of cholesterol in human cells. The effectiveness of statins in diabetes relies on the upregulation of liver LDL receptors.5 These drugs clear chylomicron remnants, intermediate-density lipoproteins, and LDL particles from the blood. The decreased LDL blood levels reduce the amount of the highly atherogenic oxidised-LDL and glycated-LDL as a consequence.32
The effectiveness of statins at reducing cardiovascular disease, all-cause mortality, and cardiovascular mortality has been observed in several clinical trials.10 They are generally well tolerated; the most common adverse effects of statins are myalgias, which can, in rare cases, lead to rhabdomyolysis and increased levels of liver enzymes.
Moderate-intensity statin therapy (a 30–50% reduction target from pretreatment LDL-C level) is recommended in primary prevention for diabetic patients >40 years. Secondary prevention therapy with high-intensity statins (a >50% reduction target from pretreatment LDL-C level) should always be recommended (Table 1).10,24
Statin treatment raises glycaemia, with a subsequent increase in the incidence of T2DM, particularly in patients with a predisposition to the condition.28,30 This effect correlates with statin potency dose33 and with its hydrophilic (i.e., rosuvastatin) or lipophilic (i.e., atorvastatin) nature. Lipophilic statins are more diabetogenic because they can penetrate extrahepatic cell membranes, such as beta cells and adipocytes, while hydrophilic statins are more hepatocyte-specific. A high hepatoselectivity translates into minimal interference with cholesterol metabolism in tissues other than the liver and, consequently, in less diabetogenicity.34 However, it must be highlighted that the benefits of lowering LDL-C levels outperform the risks of worse glycaemic control. Pitavastatin has a number of pleiotropic effects that reduce the metabolic changes associated with adiposity and improve glucose metabolism (suppressed GLUT4 expression, for example), which distinguishes it from other statins.30,35
Ezetimibe can be used in combination with statins in patients who do not achieve the therapeutic target. This drug limits dietary cholesterol absorption and the reabsorption of bile cholesterol by blocking the protein responsible for the transportation of cholesterol in the small bowel.36 It can be used for patients with hepatic or renal dysfunction. The IMPROVE IT trial37 demonstrated that the association of statins plus ezetimibe in diabetes patients resulted in a superior reduction of major adverse cardiovascular events over simvastatin therapy alone.
Fibrates reduce hepatic synthesis of VLDL by activating the peroxisome proliferator-activated receptor-alpha and increasing the activity of lipoprotein lipase that hydrolyses TG in lipoproteins. These drugs decrease TG levels, modestly increase HDL levels, and reduce total cholesterol but, in some patients, there is an increase in LDL levels because the treatment accelerates the degradation of VLDL to LDL.38 The FIELD study39 showed that fenofibrate slows progression of diabetic retinopathy and a 11% reduction of all CVD events has been reported.40 Likewise, gemfibrozil led to a significant reduction in CVD events during a 5-year follow-up period in the VA-HIT trial;41 however, the absolute benefits in primary prevention are modest and its use in monotherapy in this setting is not recommended.42 Trials exploring the use of fibrates in combination with statins have failed to show positive results, except in those with elevated TG (>200 mg/dL) or low HDL-C (<40 mg/dL).43 These drugs also increase the risk of gallstones; therefore, they are not recommended in patients with history of biliary colic. They have also been associated with an increased risk of rhabdomyolysis.44 Monotherapy with fibrates should be discouraged because there is no evidence of a reduction in all-cause mortality.
Bile acid sequestrants (BAS) act in the gastrointestinal tract, exchanging chloride anions with anionic bile acids and binding them in a resin matrix. As a result of this loss of bile acids, more cholesterol (LDL) is converted to bile acid in the liver, lowering cholesterol levels.5 There are four BAS available for the treatment of hypercholesterolaemia: colestipol, cholestyramine, colestilan/colestimide, and colesevelam.45 These drugs are poorly tolerated due to frequent gastrointestinal side effects.46 Apart from lipid-lowering effects, some older trials found an effect in lowering blood glucose and glycosylated haemoglobin levels (e.g., the GLOWS47 trial). There is a paucity of evidence evaluating the impact of BAS on cardiovascular outcomes (i.e., the CPPT48), but the available data suggest positive results. Further studies are needed to evaluate the impact of BAS in combination with statin therapy on cardiovascular morbidity and mortality.49
Omega-3 fatty acids have little effect on HDL-C or LDL-C but they can lower TG levels and be used in combination with statins and fibrates.38 However, no efficacy has been proven for the prevention of cardiovascular events in patients with glucose metabolism disorders.29
New Treatments
LDL particles are bound to LDL receptors and this complex is internalised into the cells to be dissociated by the endosome; LDL particles are eliminated by lysosomes and LDL receptors return to the surface to bind to another LDL particle. PCSK9 inhibitors block the dissociation of the LDL–LDL receptor complex and this is eliminated by lysosomes, reducing LDL receptor concentration and the clearance of LDL.50,51
Alirocumab and evolocumab are two fully human monoclonal antibodies that inhibit PCSK9 and decrease intrahepatic degradation of internalised LDL receptors, producing elevated hepatic expression of LDL receptors and a reduced level of circulating LDL-C. Both drugs have been evaluated in diverse populations, including diabetic patients, and have been proven to be safe and efficient for decreasing LDL-C concentrations in monotherapy or when given in combination with statins, with or without ezetimibe.52,53 They have also been authorised as additional therapy for patients with ASCVD or familial hypercholesterolaemia who are taking the maximally tolerated dose of statins but who require a further reduction in their levels of LDL-C.10
FOURIER54 and ODYSSEY OUTCOMES55 showed a reduced number of cardiovascular events after treatment with evolocumab and alirocumab, respectively, related to the degree of further LDL-C lowering. While neither investigated a decrease in cardiovascular death, alirocumab, but not evolocumab, showed a decreased risk of all-cause death. In addition, more injection-site reactions than placebo have been reported for both drugs, as well as a higher rate of myalgia with alirocumab when compared with placebo.56 No adverse neurocognitive events or side effects deriving from very low levels of LDL-C have been shown. In FOURIER, antidrug antibodies appeared in 0.3% of patients without neutralising antibodies.54 In another trial with alirocumab,56 1.3% of patients developed neutralising antibodies. Statins have shown a dose-dependent relationship with risk of new-onset diabetes and, moreover, mendelian randomisation studies with genetic variants in PCSK9 have shown an increased risk of diabetes;53 however, the clinical trials with PCSK9 inhibitors have not shown a higher incidence of diabetes or metabolic worsening in diabetic patients.53,56 PCSK9 inhibitors have proven to be powerful LDL-C-lowering agents; however, the need for long-term safety studies and the high associated costs are the main challenges.10,53
Possible Future Treatments
An intracellular PCSK9 inhibitor, inclisiran, is currently in development for diabetes; its main differences from other PCSK9 inhibitors are the intracellular inhibition of mRNA and administration only twice a year. In addition, an antisense oligonucleotide against ApoC-III has shown decreased plasma TG in Phase II–III trials.57 8-Hydroxy-2,2,14,14-tetramethylpentadecanedioic acid reduced total cholesterol, LDL-C, and non-HDL-C compared with placebo by modulating pathways of fatty acids and cholesterol. Saroglitazar is a dual proliferator-activated receptor-alpha/gamma agonist that reduces plasma TG, non-HDL-C, total cholesterol, VLDL, fasting plasma glucose, and HbA1c. Bempedoic acid is currently in Phase II trials and reduces LDL-C by up to 50% when combined with ezetimibe in diabetic patients.53,57
EFFECTS OF ANTIDIABETICS ON LIPID LEVELS
Table 2 provides a visual representation of the effects of antidiabetics on lipid levels. Metformin is often overlooked as a lipid-lowering agent and is generally considered only as a hypoglycaemic agent, but it has been associated with a significant decrease in plasma TG, total plasma cholesterol, LDL-C, and VLDL, as well as with a significant increase in plasma HDL-C.58 This antihyperlipidaemic effect of metformin is due to the inhibition of fatty acid release from adipose tissues. Reductions in plasma total cholesterol levels appear to be the result of decreased levels of LDL-C or VLDL. Additionally, liraglutide reduces the formation and progression of atherosclerosis; a recent study has reported for the first time that liraglutide decreases atherogenic, small, dense LDL particles known to be strongly associated with carotid atherosclerosis and CV risk.59,60
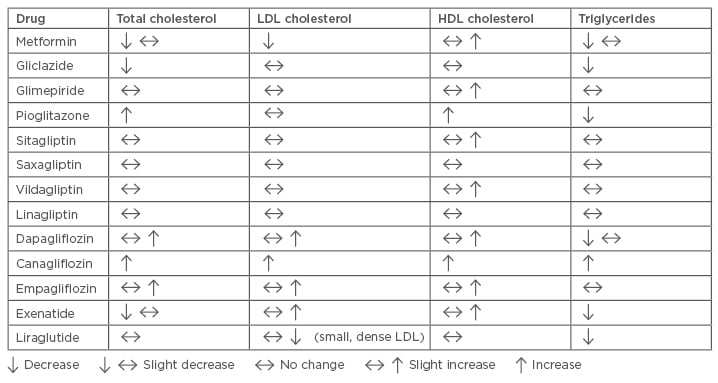
Table 2: Effects of antidiabetics on lipid levels.
HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Adapted from Soran et al.5
Other drugs used in the management of diabetes may also have unintended positive and negative effects on lipoproteins. For example, SGTL-2 receptor antagonists and pioglitazone may increase total cholesterol, LDL-C, and HDL-C, while TG levels are reduced by pioglitazone, gliclazide, exenatide, liraglutide, and dapagliflozin; however, TG levels are increased by canagliflozin.61
CONCLUSION
DD is characterised by qualitative and quantitative changes, such as increased TG, high LDL-C, and an alteration in the apolipoproteins of HDL. ASCVD is the leading cause of death in patients with diabetes and lifestyle changes remain the pillar of treatment for DD and diabetes as a whole. Statins continue to be the first-choice drugs for lowering LDL-C in patients with diabetes but new drugs, such as PCSK9 inhibitors, have been authorised as additional therapies for patients with ASCVD or familial hypercholesterolaemia who require a further reduction in their levels of LDL-C while taking their maximally tolerated dose of statins. New drugs used in the management of diabetes may also have unintended positive and negative effects on lipoproteins. Possible future treatments, including intracellular PCSK9 inhibitors, with administration twice per year, or dual proliferator-activated receptor-alpha/gamma agonists, are promising.








