Abstract
Objective: The purpose of this study was to investigate the effects of a long-term weight loss therapy in two groups (insulin resistance [IR] and non-insulin resistance [non-IR]) of obese adolescents based on metabolic profile, biomarkers of inflammation, and fibroblast growth factor-21 (FGF-21) concentrations.
Methods: Obese adolescents (15–19 years) were randomised into two groups (IR=8 and non-IR=9) and monitored through clinical, exercise training, nutritional, and psychological counselling over 1 year. Measurements of inflammatory biomarkers and FGF-21 were performed. The effects of therapy were verified by two-way ANOVA and post hoc analyses were performed (α ≤5%).
Results: A reduction in body mass, visceral fat, and an increase in adiponectin in both groups was found. Only the non-IR group demonstrated improved BMI, body fat mass, lean body mass, and waist circumference. Indeed, in the non-IR group, FGF-21 presence was positively correlated with high-density lipoprotein cholesterol and lean body mass and inversely correlated with plasminogen activator inhibitor-1 and triglycerides. In the IR group, there was a reduction in FGF-21 concentration, adiponectin/leptin ratio, insulin, total cholesterol, low-density lipoprotein cholesterol, and plasminogen activator inhibitor-1. FGF-21 was negatively correlated with delta-triglycerides, waist circumference, and low-density lipoprotein cholesterol. The IR prevalence reduced from 47% to 23.5% in the studied population.
Conclusions: Although the multicomponent clinical approach improves, in both analysed groups and in both metabolic and inflammatory states, the presence of IR resulted in a reduction in both FGF-21 concentration and adiponectin/leptin ratio. Additionally, in the IR group, FGF-21 was negatively correlated with proinflammatory markers, and in the non-IR group it was positively associated with high-density lipoprotein, suggesting its role in the control of inflammation counteracting IR. In this way, we suggest that IR can impair the anti-inflammatory effects of FGF-21. It will be helpful if these results can be confirmed in a large cohort, underlying physiological mechanisms to explore how these results can help in setting up more prospective studies.
INTRODUCTION
Obesity in adolescence urgently needs combatting, since, for the first time, the current generation will have a shorter life span than previous generations. Sedentary lifestyles and a high fat diet are the strongest predictive factors to the alarming increased prevalence of obesity.1-4 The most prevalent risk factor related to obesity in adolescents, in our clinical practice, is a state of insulin resistance (IR), leading to metabolic syndrome, diabetes, and atherosclerosis.5
The IR state is defined by the deficiency of the action of insulin, which culminates in the decrease of the absorption of glucose by the tissues, and the increase of the endogenous production of glucose by the liver. These factors result in a state of hyperglycaemia in both fasted and postprandial conditions. The association between IR and obesity occurs due to several mechanisms that act together. Among the numerous inflammatory cytokines related to obesity, it has been verified that tumour necrosis factor-alpha is capable of promoting alteration in the insulin signalling pathway.6
In the investigation the authors are currently carrying out, we consider IR to be a divider of the study group, as this condition is one of the most common metabolic alterations seen in the obese population, which is associated with cardiovascular alterations. In accordance with this comorbidity, the proinflammatory state observed in obesity contributes to this condition, especially considering the hypoadiponectinaemia, hyperleptinaemia, and elevated plasminogen activator inhibitor-1 (PAI-1) concentration.5,7-9 Moreover, previous experimental investigations have shown that fibroblast growth factor-21 (FGF-21) acts as an upstream regulator of adiponectin release, and exerts its effects on glucose metabolism and insulin sensitivity via adiponectin. It was revealed that FGF-21 increased adiponectin expression by activating peroxisome proliferator-activated receptor-gamma, and displayed various metabolic functions, mainly through adiponectin.10-12 In human studies, analogues of FGF-21 induced increases in adiponectin levels, suggesting their cardiovascular protective factor.13,14
Additionally, leptin was considered a potent metabolic modulator of FGF-21, as seen in experimental studies and research performed in vivo with cell culture.15,16 It suggested FGF-21 was an integrative hormone involved in multiple actions within mammalian organisms.15,16 The high levels of PAI-1 are associated with an increased cardiovascular risk of thrombosis. As previously described, increases in PAI-1 are undoubtedly related to IR, and the mechanisms that could explain such an increase in the metabolic disorders, related to obesity and metabolic syndrome.17-19
FGF-21, a novel hormone-like protein expressed in many tissues, has recently been described as having a key role in the ‘browning’ of adipocytes, being originally associated with increased secretions by muscle tissues after exercise in both experimental and clinical studies. Thus, FGF-21 is referred to as the thermogenic protein, promoting energy expenditure by converting white adipose tissue to brown adipose tissue as well as activating brown adipose tissue.20 In humans, the liver mostly secretes FGF-21; however, it is also released by adipocytes from both subcutaneous and visceral adipose tissue. Notably, FGF-21 has abundant adipose tissue receptors, which mediates the browning process.21 Unfortunately, an excess of circulating FGF-21 may lead to a FGF-21 resistance in obese women, hindering its action.22
In addition, FGF-21 exerts beneficial effects on metabolic homoeostasis regulated by adipokines, such as adiponectin, leptin, and resistin. In particular, FGF-21 deficiency, rather than resistance, contributes to development of IR and hypoadiponectinaemia, independently of obesity in young people.12 Moreover, the beneficial effects of FGF-21 in IR, lipid profile, and energy homeostasis have been demonstrated in clinical and experimental studies,13,14,23,24 suggesting a possible new therapeutic target for treating obesity and related comorbidities.12 However, data on the role of FGF-21 in adolescents with obesity are limited.25 In this way, we hypothesise that the IR condition present among obese adolescents could promote different metabolic responses in regard to FGF-21 concentrations and related metabolic and inflammatory effects.
Thus, the purpose of this study was firstly to investigate the effects of a long weight loss therapy in two different groups (with and without IR) of obese adolescents on FGF-21 concentration, metabolic profile, and pro/anti-inflammatory adipokines (including adiponectin and leptin) in visceral and subcutaneous adipose tissues. Secondly, this study aimed to verify if IR modulates the role of FGF-21 in obesity in a sample of obese adolescents undergoing long-term multicomponent therapy.
MATERIALS AND METHODS
Population
This preliminary study included 23 post-puberty Brazilian/Caucasian adolescents with obesity, aged 15–19 years (16.13±0.8) including both sexes (13 females and 10 males). Inclusion criteria were Tanner Stage 5,26 primary obesity, BMI, >95th percentile of the CDC reference growth charts.27 Non-inclusion criteria were the use of birth control pills, cortisone, anti-epileptic drugs, history of renal disease, alcohol intake, smoking, and secondary obesity due to endocrine disorders.
The adolescents were divided into two groups: presence of IR (n=8) and non-IR (n=9). The reasons for dropping out (n=6) of the study included financial and family problems along with school and job opportunities. No sex difference was observed in adherence rates. The study was conducted with the principles of the Declaration of Helsinki, approved by the ethics committee on research at the Universidade Federal de São Paulo (UNIFESP 152.281), Clinical Trial: NCT01358773. All procedures were clear to those responsible for the volunteers and informed consent forms were signed. All evaluations were performed at two different times (baseline: beginning, and after therapy: after 1 year of interdisciplinary weight loss therapy).
Anthropometric Measurements
Body composition and weight were measured by plethysmography scale (BODPOD equipment),28 where patients wore minimum clothing where possible. Height was measured using a stadiometer (Sanny-model ES 2030) and BMI was calculated by dividing the weight by height squared (kg/m²). Waist circumference was obtained at the midpoint between the last rib and iliac crest.
Serum Analysis
Blood samples were collected after an overnight fast in the outpatient clinic at approximately 08:00 am. After collection, the blood was centrifuged for 10 minutes at 5,000 rpm and stored at -70°C for future analyses. The materials used for collection were disposable and adequately labelled. Blood was collected by a skilled and qualified technician. A lipid profile was obtained from the blood. IR was assessed according to the Homeostasis Model Assessment of Insulin Resistance Index (HOMA-IR). The HOMA-IR was calculated as the product of the fasting blood glucose and the immunoreactive insulin levels (fasting blood glucose [µU/mL] × fasting blood glucose [M/22.5]). All of the variables were analysed using a commercial kit (CELM, Barueri, Brazil). Total cholesterol, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) were analysed using a commercial kit (CELM). The reference values adopted were insulin (<20 µU/mL), HOMA-IR (>3.16), total cholesterol (<170 mg/dL), LDL cholesterol (<130 mg/dL) as previously described by Schwimmer et al.29 The PAI-1, adiponectin and leptin were measured by enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (Minneapolis, Minnesota, USA), and FGF-21 concentrations were measured using a commercially available MULTIPLEX assay (EMD Millipore, Burlington, Massachusets, USA; HMHMAG-34K).30 For this study, leptin data were analysed according to reference values.31
Visceral and Subcutaneous Adiposity Measurements
Ultrasound measurements of the visceral and subcutaneous fat were taken. All abdominal ultrasonography procedures were performed by the same blinded diagnostic imaging specialist, who used a 3.5 MHz multifrequency transducer (broadband) before and after the intervention. Ultrasound-determined subcutaneous fat was defined as the distance between the skin and external face of the rectus abdominis muscle; visceral fat was defined as the distance between the internal face of the same muscle and the anterior wall of the aorta. The intra-examination coefficient of variation for ultrasound was 0.8%. The cut-off points for the definition of visceral obesity by ultrasonography were based on previous methodological descriptions produced by Ribeiro-Filho et al.32
Descriptive Methodology of Multicomponent Weight Loss Therapy
The study sample was completed by adolescents with obesity within both sexes (n=17). An interdisciplinary group of health professionals conducted all sessions. At three-times per week during 2 non-consecutive hours per day, the adolescents participated in supervised therapy for physical exercise practice, nutrition, and psychological attendances over 1 year (Figure 1).
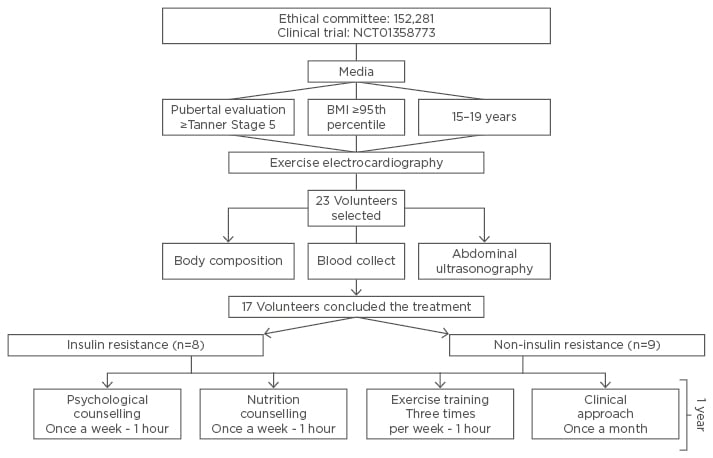
Figure 1: Diagram of interdisciplinary therapy.
Clinical Approach
The adolescents with obesity visited the endocrinologist with their parents once each month. The doctor monitored and evaluated all clinical examinations, including the initial medical history and a physical examination of blood pressure, cardiac frequency, and body mass. The adolescents were checked for their adherence to all interdisciplinary therapies (Figure 1).
Aerobic Plus Resistance Training Intervention
The adolescents followed a combined physical exercise training therapy programme. The protocol was performed three-times per week for 1 year and included 30 minutes of aerobic training plus 30 minutes of resistance training per session. The subjects were instructed to reverse the order of the physical exercises (aerobic and resistance) at each training session. The aerobic training consisted of running on a motor-driven treadmill (Life Fitness: model TR 9700HR) or bicycle at a cardiac frequency intensity representing the ventilatory threshold I (±4 bpm), which was determined by the results of an initial oxygen uptake test for aerobic exercises (ergospirometry). The physical exercise therapy and resistance training were based on the guidelines from the American College of Sports Medicine (ACSM) (Figure 1).33,34
Nutrition Counselling
Energy intake was set at levels recommended by the dietary reference intake for subjects with low levels of physical activity of the same age and sex.35 Once a week, adolescents received dietetic lessons covering the topics related to a healthy eating pattern, including the food pyramid, diet record assessment, weight loss diets and fad diets, food labels, dietetics, fat-free and low-calorie foods, and other related topics. All participants had monthly, individual consultations (Figure 1).
Psychological Counselling
All adolescents participated in weekly psychological orientation group sessions based on the psychodynamic approach with a trained psychologist. Individualised psychological therapy was recommended when it was necessary according to their psychological assessment (Figure 1).
Statistical Analysis
Statistical analysis was performed using the program STATISTICA, version 7.0 for Windows Vista. The adopted significant value was α≤5%. Data normality was verified with the Shapiro Wilk test. Parametric data were expressed as mean ± standard deviation (SD), and non-parametric data were expressed as median, minimum, and maximum values. The effects of therapy were verified by two-way ANOVA, Tukey’s post hoc analysis for parametric data, and the Wilcoxon test for non-parametric data. Correlation analyses were established through the Pearson’s test for parametric data and Spearman’s test for non-parametric data (α≤5%).
RESULTS
All Groups
At baseline, there was no difference observed between groups for body mass and BMI (Table 1A). FGF-21 concentration positively correlated with lean body mass and negatively correlated with waist circumference (Table 1B).
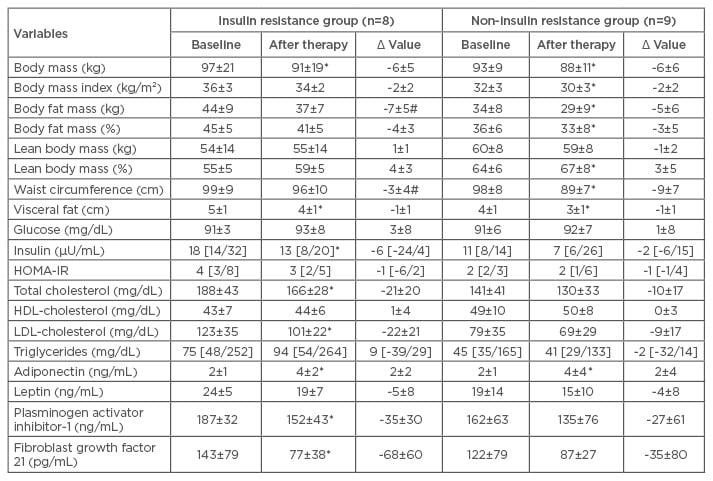
Table 1A: Long-term effects of multicomponent therapy in obese adolescents.
Statistical significance: p≤0.05. Reference values: glucose (60–110 mg/dL); insulin (<20 μU/mL); HOMA-IR (>3.16); QUICKI (>0.339) as previously described by Schwimmer et al.29
*Statistical difference after therapy in the same group; #statistical difference after therapy between groups.
HDL: high-density lipoprotein; HOMA-IR: Homeostasis Model Assessment Insulin-Index Resistance; LDL: low-density lipoprotein.
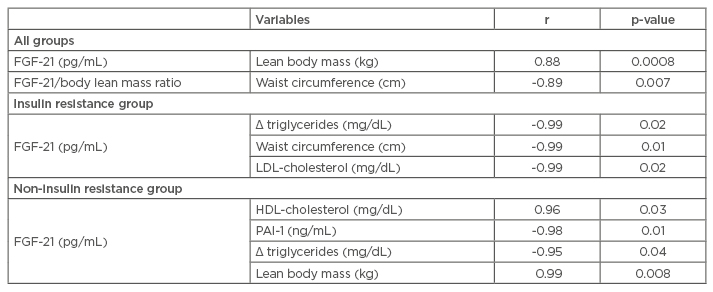
Table 1B: Correlations analysis.
Statistical significance: p≤0.05.
FGF-21: fibroblast growth factor 21; HDL: high-density lipoprotein; LDL: low-density lipoprotein; PAI-1: plasminogen activator inhibitor-1.
Effects of Interdisciplinary Therapy in the Insulin Resistance Group
In the IR group, body mass, visceral fat, insulin concentration, total cholesterol, LDL cholesterol, PAI-1, FGF-21 (Figure 2A), FGF-21/lean body mass ratio, and adiponectin/leptin ratio (Figure 2B) were reduced. Adiponectin concentration was increased. In the correlation analyses, FGF-21 negatively correlated with delta-triglycerides, waist circumference, and LDL-cholesterol. The IR prevalence reduced from 47% to 23.5% in the study sample.
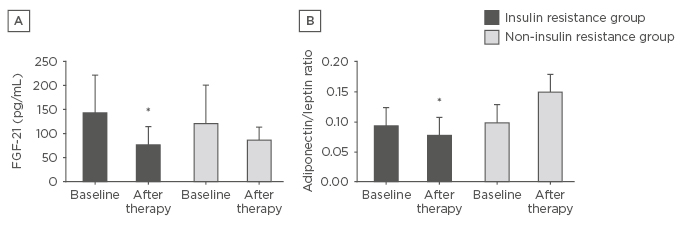
Figure 2: Effects of the interdisciplinary weight loss therapy on FGF-21 and adiponectin/leptin ratio.
A) Effects of the interdisciplinary weight loss therapy on FGF-21 in both analysed groups; B) Effects of the interdisciplinary weight loss therapy on adiponectin/leptin ratio in both analysed groups.
*Statistical significance.
FGF-21: fibroblast growth factor-21.
Effects of Interdisciplinary Therapy in the Non-Insulin Resistance Group
In the non-IR group, there was an improvement in body mass, BMI, body fat mass (kg and %), body lean mass (%), waist circumference, and visceral fat. In this group, adiponectin concentration was increased. In the non-IR group, FGF-21 positively correlated with HDL-cholesterol and lean body mass, and inversely correlated with PAI-1 and triglycerides.
DISCUSSION
The main purpose of the present investigation was to analyse the long-term effects of weight loss therapy on FGF-21 concentration, and whether this hormone can modulate the metabolic profile and pro/anti-inflammatory adipokines in obese adolescents with and without IR. Therefore, the most important finding in the present study was that in the IR group, FGF-21 negatively correlated with delta-triglycerides, waist circumference, and LDL-cholesterol. This is an important finding because, in recent years, it has been reported that FGF-21 may exert cardio-protective effects.36 Only the non-IR group demonstrated an improvement in BMI, body fat mass, lean body mass, and waist circumference. Indeed, in the non-IR group, FGF-21 was positively correlated with HDL-cholesterol and with lean body mass. In accordance with our results, previously Li et al.12 showed that reduced FGF-21 levels were negatively correlated with adiposity measures, such as BMI and waist circumference, in the obese paediatric population.
The present study, unfortunately, was not able to demonstrate a statistical dependence of changes in the biomarkers of inflammation with the body weight change (a reduction of approximately 6 kg), according to the statistical analyses applied. It is relevant to note that improvements in adiponectin concentration occurred in both groups. Although, the PAI-1 was ameliorated only in the IR group, after weight loss therapy.
These data are in accordance with a previous study published by the same group, where we found that a low-to-moderate weight loss (>5.8–≤10.9 kg) induced metabolic changes, including improvement of HOMA-IR index, adiponectin/leptin ratio, and reductions in PAI-1 concentration. It was observed in this previous investigation that changes in body weight were considered an important determinant of changes on inflammatory profile in obese adolescents.37
It is important to note that, in these results, only the non-IR group was FGF-21 inversely correlated with PAI-1 and triglycerides. PAI-1 is expressed in many tissues, including visceral and subcutaneous adipose tissue depots, where adipose tissue- resident macrophages are the primary source of the cytokines in these depots. PAI-1 is the prime regulator of fibrinolysis, and subjects with increased levels of PAI-1 are predisposed to thrombotic events. Obese patients with and without Type 2 diabetes mellitus have elevated levels of PAI-1 as compared to lean individuals, and this is closely linked with IR,38-40 supporting the data observed in the present study.
Another interesting result confirmed in the present investigation is that only in the IR group was FGF-21 concentration significantly reduced (Table 1A). Although it has been previously shown that in obese children and adolescents the serum FGF-21 level was higher in the IR group,41 neither in the present study nor the study performed by Cheung and Deng42 were these results confirmed. A strong association was shown between reduced FGF-21 levels, IR, and metabolic syndrome in obese paediatric patients.12 In addition, FGF-21 has been demonstrated to alleviate IR, not only in adipose tissue but also in the liver and muscle tissues.43,44 In fact, our data suggest a state of FGF-21 resistance in obese adolescents with IR, corroborating with previous research in obesity.16 However, weight loss could reverse this resistance, a hypothesis that needs to be explored in a large cohort of obese adolescents.
The IR prevalence reduced from 47% to 23.5% in the present study, which partially explains the main effects of multicomponent therapy in enhancing the metabolic and anti-inflammatory effects in response to this approach. Moreover, both analysed groups present a reduction in body mass and visceral fat, showed a positive correlation between FGF-21 and lean body mass, and a negative correlation with waist circumference, contributing to our understanding of the role of FGF-21 in energy expenditure and ‘browning’.45,46
FGF-21-like adiponectin has been considered to be metabolically protective against atherosclerosis. Additionally, it is important to note that in both groups, improvements in the hypoadiponectinaemia was shown in response to weight loss therapy. These data may contribute to a greater understanding on the FGF-21 effects in atherosclerosis, mediated by adiponectin secretion.20,47,48
Interestingly, the triglycerides, LDL-cholesterol, and waist circumference, were confirmed as a negative independent predictor of FGF-21 in the IR group. Inversely, HDL-cholesterol and lean body mass were positively correlated with FGF-21 in the non-IR group. In addition, PA1-1 and triglycerides negatively predict FGF-21 in this group.
Together, all these findings observed in the present investigation reinforces the link between IR and atherosclerosis, since reduced adiponectin/leptin ratio (a potent biomarker of anti-inflammatory state), was seen only in the IR group, leading to a hypothesis that IR can impair the anti-inflammatory effects of FGF-21. In the present study, an inverse correlation between FGF-21 and PAI-1, and positive correlation between FGF-21 and HDL-cholesterol, was observed when IR was not present in obese adolescents.
Although the multicomponent clinical approach has improved, in both analysed groups and in both metabolic and inflammatory states, the presence of IR resulted in a reduction in both FGF-21 concentration and adiponectin/leptin ratio. Additionally, in the IR group, FGF-21 was negatively correlated with proinflammatory markers, and in the non-IR group, this hormone was positively associated to HDL and negatively with pro-thrombotic and inflammatory risks, suggesting its role in the control of inflammation, counteracting the state of IR in obese adolescents. This is important to help health professionals consider that both early detection and treatment of obesity in adolescents could be the best tool to prevent future increases in comorbidities and healthcare costs in younger age.49
In this way, these results demonstrate interesting preliminary data that can be applied in clinical practice, considering the relevant activity of FGF-21 on the metabolic and inflammatory profile of obese adolescents in the presence of IR state. It is important to reinforce that, despite a small sample size in this study, the data presented promising results and was confirmed through statistical analyses (α=0.05), as considered and discussed in this article. Future investigations need to confirm these results as there are limitations to this study, including the small sample size and differences between sexes that were not investigated.
Additionally, future studies may explore the clinical evaluation of therapeutic action of exogenous FGF-21 administration; in particular, studies in treating diabetes, metabolic syndrome, atherosclerosis, and obesity are warranted. Finally, it will be helpful for further investigations to look into the underlying physiological mechanisms, to explore how this study’s results can help in setting up more prospective studies.








