Abstract
Gestational diabetes mellitus (GDM) is a pervasive metabolic disorder associated with a spectrum of long-term adverse outcomes. Recent evidence indicates that women with GDM have a heightened subsequent risk of kidney disease. Persistent factors, both pre-gestational and postpartum, can contribute to these adverse outcomes years after a GDM pregnancy. Metabolic features such as insulin resistance, subclinical inflammation, and endothelial dysfunction can lead to enduring microvascular alterations, ultimately resulting in long-term renal complications. The insulin resistance and beta cell dysfunction that develop during GDM are chronic and progressive, increasing the risk of Type 2 diabetes mellitus, hypertension, and dyslipidaemia, all risk factors for chronic kidney disease (CKD).
While few studies have specifically investigated the independent association between GDM and subsequent renal dysfunction, a recent study examining the adverse pregnancy outcomes and long-term risk of CKD identified GDM as one of the independent risk factors. The findings of this review strongly recommend that women who experience adverse pregnancy outcomes like GDM during their reproductive years should be well-informed about their long-term risk of kidney disease. This knowledge is essential for early preventive actions and follow-up care. In future, cardiometabolic surveillance and risk modification strategies in clinical practice are necessary to prevent maternal renal complications among women with a history of GDM.
Key Points
1. Gestational diabetes mellitus (GDM) affects a significant portion of pregnancies worldwide, posing health risks to both mother and child. It is linked to increased long-term renal morbidity, significantly impacting quality of life.
2. This review examines the evidence supporting the association between GDM and the development of kidney disease, highlighting potential contributing factors
3. Women who have experienced GDM should be aware of their elevated risk for kidney disease and take proactive steps to prevent it. Regular monitoring and appropriate interventions are crucial to mitigate the morbidity and mortality associated with this condition.
INTRODUCTION
Gestational diabetes mellitus (GDM) is a rapidly growing global health issue and one of the most common metabolic complications in pregnancy.1 GDM is defined as any degree of glucose intolerance first recognised during pregnancy. It arises when the insulin response is insufficient due to underlying insulin resistance or inadequate insulin secretory capacity caused by placental hormones as pregnancy progresses.1 According to the International Diabetes Federation (IDF), 16.7% of live births to women were complicated by hyperglycaemia during pregnancy, with 80.3% of these cases attributed to GDM.2 An increase in prevalence of GDM is driven by multiple factors, including patients who are overweight or obese, older age, family history of diabetes mellitus, previous history of GDM, excessive weight gain during pregnancy, and habitual smoking.2 Even though GDM exists as a transient disorder during pregnancy, accumulating evidence supports that GDM is associated with subsequent Type 2 diabetes (T2D), hypertension, dyslipidaemia, vascular dysfunction, and cardiovascular disease which are risk factors of renal impairment.3-7
Chronic kidney disease (CKD) is a rapidly growing public health problem associated with increased morbidity and mortality.8 GDM is one of the metabolic diseases associated with long-term kidney disease.9,10 Transient hyperglycaemia, which is limited to gestation, may support the perception of GDM as a medical complication of pregnancy, while the glycaemic impact of this complication extends well beyond the gestational time period. Health implications of GDM beyond the gestational period are well studied, and most attention is paid to the increased risk of recurrent GDM, impaired glucose tolerance, and diabetes mellitus.11-13 Women with GDM have also demonstrated an increased risk for metabolic syndrome, vascular endothelial dysfunction, dyslipidaemia, and cardiovascular events.11,14-19 However, relatively few studies investigated the relationship between GDM and the long-term risk of complications such as renal disease.6,20-23 As the prevalence of GDM has been increasing globally, the burden of long-term adverse maternal outcomes such as renal disease also substantially increased.9,24,25 Moreover, it seems plausible to speculate the association between GDM and long-term development of kidney disease due to its significant impact on quality of life, health care cost, morbidity, and mortality associated with it. 1,26 Hence, it is important to study the effect of GDM on the function of the kidneys during the gestational time period and following pregnancy.6,20 This review provides an overview of the impact of gestational diabetes on the long-term risk of kidney disease and the potential factors associated with it.
The potential mechanism behind the association between GDM and kidney disease is composed of many factors involved in the pre-gestational period and persisting in the postpartum period. Insulin resistance, subclinical inflammation, epigenetic changes, environmental factors, and lifestyle modifications are some of the factors associated with this.6,22,23 Altered glucose metabolism in GDM disrupts the maternal vasculature due to the deleterious effects of potential mediators such as reactive oxygen species, advanced glycation end products, and pro-inflammatory cytokines, which in turn activate pathological signalling pathways. Oxidative stress-associated changes in the placenta also contribute to accelerating these pathological modifications.27,28 Renal morphological changes such as thickening of the glomerular basement membrane, increase in glomerular size, and mesangial matrix expansion are also noticed in GDM models (Figure 1).29 Based on the literature findings, women with GDM show early metabolic changes such as endothelial abnormalities even before glucose intolerance occurs.30 In addition, GDM women are also susceptible to subclinical inflammation and vascular changes such as increased peripheral vascular resistance and impaired endothelium-dependent vasodilatation, both of which lead to renal dysfunction independently.7,30 Probable development of metabolic syndrome, T2D, hypertension, dyslipidaemia, and other cardiometabolic disorders after GDM is also inevitable.5,11,31,32 Collectively, all these findings indicate that the metabolic impact of GDM may persist beyond the gestational period and adversely affect long-term renal function.
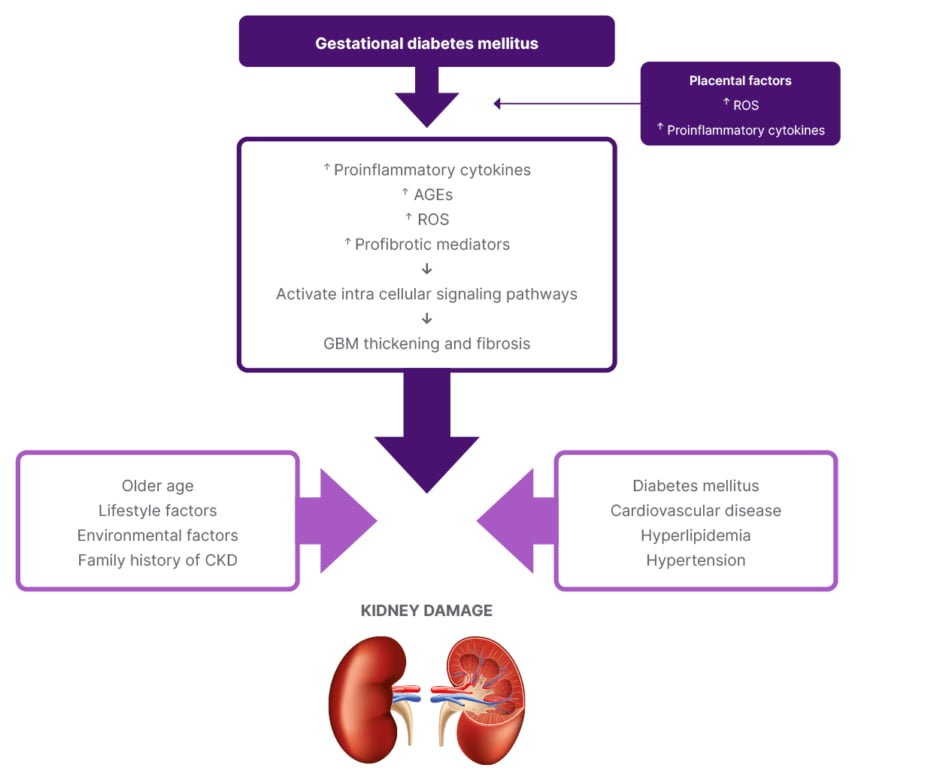
Figure 1: The potential metabolic changes associated with gestational diabetes mellitus in developing kidney damage and the major risk factors associated with it.
AGE: advanced glycation end products; CKD: chronic kidney disease; GBM: glomerular basement membrane; ROS: reactive oxygen species.
The potential impact of GDM on kidney dysfunction was studied among women with a history of GDM, and a greater prevalence of microalbuminuria was observed to be more frequent.21,22,33 In addition to this, elevated levels of glomerular filtration rate, an early sign of glomerular hyperfiltration and renal impairment, was noticed among women with GDM after 9–16 years of follow-up.34 More importantly, patients with recurrent episodes of GDM reported more risk for future renal morbidity.23 Interestingly, Aboriginal women with a previous history of GDM, a high GDM-risk population, observed a remarkably high risk of developing CKD and end-stage renal disease.10 Recently, an independent association of GDM and incident kidney disease was also observed, and it was also reported that pregnant women with severe metabolic dysfunction are at high risk for future renal disease.6, 20,35
Recently, a systematic review and meta-analysis indicated that exposure to GDM has an elevated risk of long-term kidney disease.36 All these findings indicate the independent involvement of GDM in developing kidney disease.
This review also observed the potential involvement of ethnicity/race in developing renal disease. Based on the current literature, African-American women who are obese with prior GDM have a high risk for CKD.21,22 This could be due to differences in the severity of GDM, the development of increased blood pressure after GDM, as well as the lactation pattern.37,38 A genetic study of African-American women with GDM shows that there are genes within or flanking the major histocompatibility complex that play a role in the aetiology of diabetes mellitus, insulin resistance, hypertension, and microalbuminuria.38 Furthermore, lower rates of breastfeeding and more severe complications of GDM are also reported among this population.39,40
Interestingly, a low incidence of renal outcome was also noticed among women with previous histories of GDM.41,42 This could be due to early postpartum assessment as well as a relatively young and healthy population with less involvement of potential confounding factors. Moreover, previous studies examining the relationship between GDM and long-term CKD exhibited substantial heterogeneity due to varying criteria for GDM diagnosis, differences in study design and follow-up duration, and sample size. Table 1 presents recent findings on the long-term risk of kidney disease following GDM.
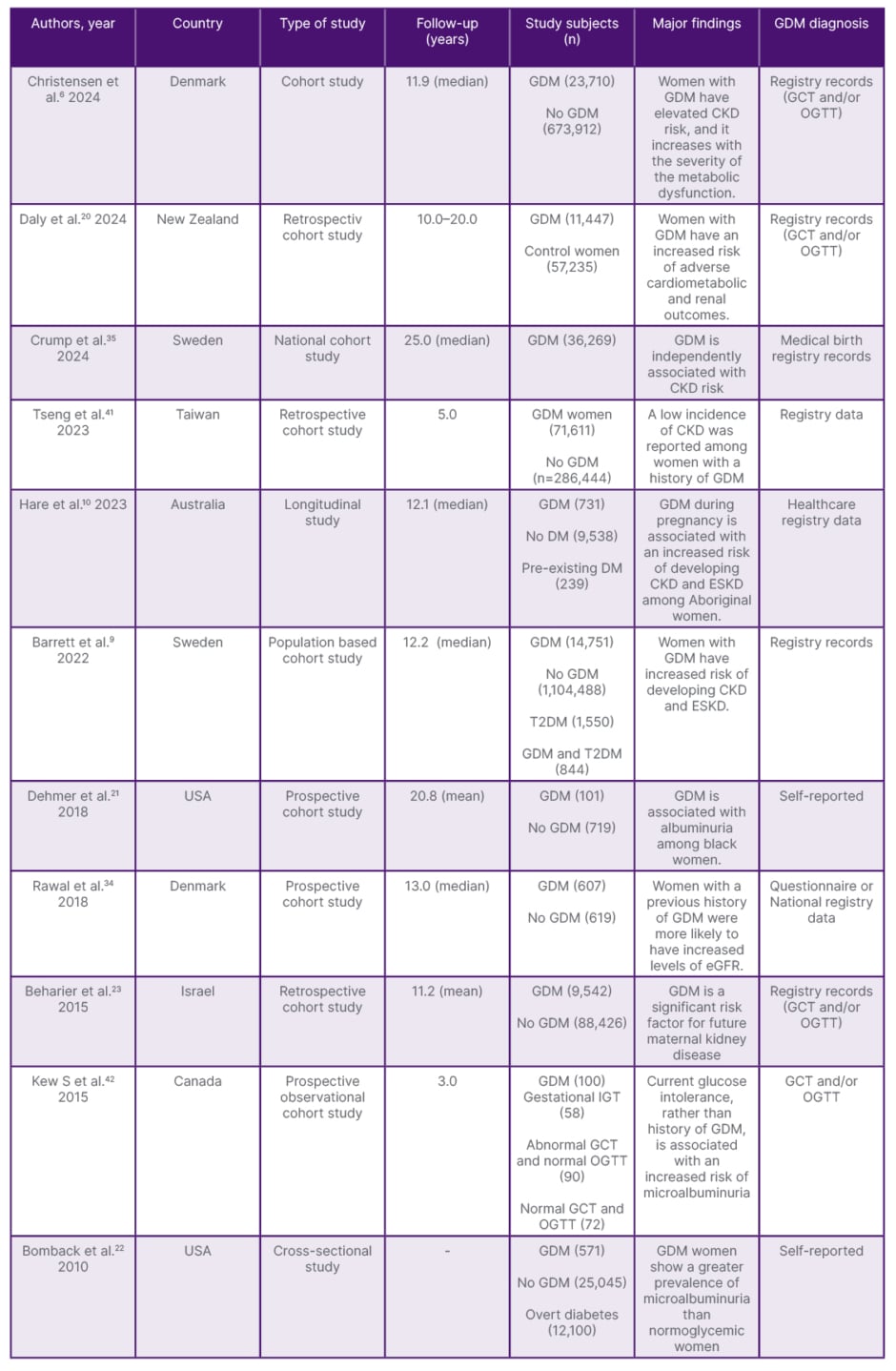
Table 1: Recent findings of long-term risk of kidney disease after gestational diabetes mellitus.
CKD: chronic kidney disease; DM: diabetes mellitus; ESKD: end stage kidney disease; GCT: glucose challenge test; GDM: gestational diabetes mellitus; IGT: impaired glucose tolerance; OGTT: oral glucose tolerance test; T2DM: Type 2 diabetes mellitus.
RISK FACTORS FOR GESTATIONAL DIABETES MELLITUS-RELATED KIDNEY DAMAGE
Even though gestational hyperglycaemia resolves after pregnancy, beta cell dysfunction and insulin resistance persist for many years after index pregnancy. Chronic progressive beta cell dysfunction, where the degree of decline in beta cell function depends on the severity of gestational dysglycaemia, is likely to be the pathophysiologic defect that leads to the manifestation of long-term diabetic risk.43,11 Diabetes mellitus is a strong risk factor for the development of kidney disease.44
Moreover, women with a history of GDM alone, even in the absence of subsequent overt diabetes, may increase the risk of future cardiovascular and kidney disease.22 Hypertension is another important risk factor for the development of renal disease, and women exposed to GDM had an increased risk of developing hypertension.31 Hypertension progresses to renal disease through the involvement of insulin resistance, which leads to impairment in the nitric oxide pathway and activates the MAPK pathway; this causes a proinflammatory state, and the increased water and sodium retention induces a hypertensive state.45-47 Furthermore, changes in the intraglomerular capillary pressure also lead to glomerulosclerosis and renal disease.48 Another independent risk factor is hyperlipidaemia, which is associated with postpartum dyslipidaemia among women with a history of GDM.5,49 Hyperlipidaemia progresses to renal disease through reabsorption of fatty acids and phospholipids through tubular epithelial cells, leading to tubulointerstitial inflammation, lipoprotein accumulation in the glomerular mesangium, and glomerulosclerosis.50 Moreover, other risk factors, such as heart disease, family history of CKD, inherited kidney disorders, older age, smoking, obesity, and socioeconomic status, can also influence the development of kidney disease.20,32
In conclusion, this literature review provides an overview of GDM and long-term kidney disease and associated potential factors according to the most recent literature findings. Based on the review findings, few studies have specifically investigated the independent association between GDM and subsequent renal dysfunction and identified GDM as one of the independent risk factors. GDM-associated metabolic changes as well as the involvement of persistent factors, both pre-gestational and postpartum, can contribute to the development of renal disease. Women with GDM represent a high-risk group for renal disease, and regular monitoring is required to prevent the morbidity and mortality associated with it. Recognition of predisposition to kidney disease among women with GDM may provide an opportunity to undertake lifestyle modifications and encourage regular screening and early intervention to minimise the harmful end-organ damage.
PREVENTION AND MANAGEMENT OF GESTATIONAL DIABETES MELLITUS-RELATED KIDNEY DAMAGE
Women who experience GDM have an elevated risk of long-term kidney disease. It is widely recommended that women with a history of GDM undergo postpartum glucose tolerance screening within 6 months after delivery to identify the risk of developing T2D, a risk factor for the development of renal impairment.51 Moreover, attention should be given to cardiometabolic risk factors such as dyslipidaemia, hypertension, and obesity to reduce the future long-term risk for kidney disease. Adverse pregnancy outcomes, especially GDM, should be added to renal risk prediction models to reduce the burden of kidney disease by supporting primary and secondary prevention strategies. Lifestyle interventions such as diet and physical activity may help to reduce the burden of long-term maternal complications. There are certain patient factors that address the poor adherence rates to postpartum screening, such as adjustment to a new baby, lack of time, emotional stress, lack of motivation, financial barriers, and lack of knowledge about GDM.51
FUTURE PERSPECTIVE
GDM is one of the chronic cardiometabolic conditions that is first identified in pregnancy and carries long-term implications. The broader life course perspective of GDM enhances the recognition of the need for focusing future studies on long-term maternal complications rather than short-term pregnancy outcomes. In future, cardiometabolic surveillance and risk modification strategies can be used in clinical practice to enable the prevention of maternal renal complications among women with a history of GDM. Further research is needed to identify effective risk-reduction interventions. Based on the reviewed findings, women with previous GDM complicated pregnancy may represent a high-risk group that requires regular monitoring for early-stage renal impairment, which may help the clinician initiate the treatment to delay or prevent the progression of the disease.







