Abstract
The incidence of diabetes has been increasing and, in parallel, so has the incidence of females in their childbearing years with diabetes. Preconception care is important in females with diabetes due many factors related to fertility, blood glucose control, and complications. For example, many individuals with Type 2 diabetes are obese, which can affect contraceptive efficacy, fertility, and fetal growth. Additionally, patients with all types of diabetes are at risk for disordered eating, which can be harmful to a developing fetus. Both hypoglycaemia and hyperglycaemia are known to increase the risk of adverse maternal and neonatal outcomes, including during the first trimester, when many females are not aware of pregnancy. Additionally, individuals with diabetes are at risk for complications, such as atherosclerotic cardiovascular disease, nephropathy, retinopathy, and neuropathy, that can lead to complicated pregnancies. Importantly, several of the medications used to control blood glucose, and manage diabetes complications, are not recommended for use during pregnancy due to potential fetal harm. For these reasons, females with diabetes in their childbearing years are encouraged to utilise reliable contraception, so that pregnancies can be planned, or should be treated with medications with low teratogenicity potential. Thus, the preconception care of females with diabetes is complex, and the increasing prevalence of this patient population warrants greater awareness among clinicians. This narrative review summarises the current standard of preconception care for individuals with diabetes, including the management of contraception, weight, blood glucose, hypertension, and dyslipidaemia.
Key Points
1. The incidence of individuals with diabetes in their childbearing years has been increasing. Preconception care in these individuals is complex due to factors related to fertility, blood glucose control, and complications. This narrative review summarises the current standard of preconception care for individuals with diabetes, including the management of contraception, weight, blood glucose, hypertension, and dyslipidaemia.
2. Family planning is important for individuals with diabetes to minimise the risk of pregnancy complications. The use of reliable contraception is important, such that first-line medications to prevent diabetes complications can be safely used.
3. Once a decision to attempt pregnancy has been made, individuals should be transitioned to treatment that is safer during gestation. Patients can be shifted to safer medications for blood pressure, medications for dyslipidaemia can be temporarily stopped, and insulin can be initiated if needed, to reach blood glucose goals. The involvement of a multidisciplinary healthcare team is recommended.
INTRODUCTION
According to the World Health Organization (WHO), the number of people with diabetes has quadrupled since the 1980s, with estimates of over 420 million people diagnosed.1 The International Diabetes Federation (IDF) estimates that over 530 million adults have diabetes, with many individuals unaware that they have it.2 The percentage of individuals with Type 1 diabetes has remained stable, but there are greater numbers of individuals with Type 2 diabetes secondary to an ageing population, sedentary lifestyle, and increasing worldwide obesity.2 Similarly, there are more individuals with diabetes in their childbearing years worldwide, due to more individuals with Type 2 diabetes, and advances in reproductive technology leading to higher maternal ages, since the incidence of diabetes increases with age.3 Within this review, the term female will be used to discuss all individuals who are potentially able to become pregnant, and is intended to be inclusive of gender-diverse individuals with diabetes in their childbearing years. The term male will be used to discuss individuals who produce sperm, and is intended to be inclusive of gender-diverse individuals that may utilise other terms to describe themselves.
Considerations of preconception care encompass decades of a female’s life, since many pregnancies are unplanned, and contraception is not 100% reliable. A United Nations Population Fund (UNFPA) report estimates that close to half of all pregnancies worldwide are not planned.4 Therefore, anyone of childbearing age (including pre-teens and adolescents) should regularly receive age-appropriate counselling regarding pregnancy planning. In individuals with diabetes, the complexity of care increases multifold. Access to counselling, as well as the involvement of a multidisciplinary team that may include a primary care provider, endocrinologist, obstetrician/gynaecologist, registered dietician, pharmacist, and diabetes care and education specialist, is recommended.5 General guidelines for preconception care that apply to individuals without diabetes should be followed for individuals with diabetes (Table 1).5,6

Table 1: General preconception care recommendations.5,6
CONTRACEPTION AND FERTILITY
Family planning is important for patients with diabetes due to the associated increased risk of pregnancy complications.7,8 Planning pregnancies reduces the risk of complications during pregnancy and peripartum in patients with diabetes.5 If pregnancy is not desired at the current time, patients should use reliable contraception when engaging in sexual activity with reproductive potential.5 If pregnancy is desired, patients should work with their care team to optimise medications, lifestyle, and health outcomes, including reaching an optimal HbA1c, before conception.
Choosing contraception should be done in a patient-specific manner, with considerations for efficacy rates with typical use; safety, including precautions and contraindications; and the individual’s preferred dosage form.7,8 Long-acting reversible contraception, which includes intrauterine devices (IUD) and subcutaneous progestin implants, should be considered whenever practical, due to their high efficacy rating, limited potential for nonadherence, and relative safety profiles.5,7 Patients should remain on contraception until they are ready to conceive.
The UK Medical Eligibility Criteria (UKMEC) and United States Medical Eligibility Criteria (USMEC) ranks the safety of contraceptive products on a 4-point scale.7,8 A category of 1 represents no known restrictions on the use of a contraceptive product in designated populations.7,8 A ranking of 4 means the use of that contraceptive product would pose an unacceptable health risk to the user, or is contraindicated.7,8 Scores of 1 or 2 indicate the contraceptive product is typically recommended in that population, and the advantages outweigh any theoretical or proven risks.7,8 For individuals with a history of gestational diabetes, all contraceptive products are ranked as category 1, and are safe to use.7,8 Additionally, copper-containing IUDs are ranked as category 1 for patients with diabetes, regardless of the type of diabetes or the presence of complications.7,8 All other contraception, including progestin-containing IUDs, implants, depot medroxyprogesterone acetate (DMPA), progestin-only pills, and combined hormonal contraceptive (CHC) products (pill, ring, or patch) are ranked as category 2, with two notable exceptions. CHCs are ranked as a category 3 in the UKMEC, and category 3 or 4 (depending on the severity of complications) in the USMEC for patients with microvascular complications of diabetes (nephropathy, retinopathy, or neuropathy), diabetes with another vascular disease, or diabetes of greater than 20 years duration, due to the higher risk of thrombosis. The other notable exception is that, for this same patient population, UKMEC ranks DMPA as a category 2, whereas the USMEC ranks it as a category 3 due to its metabolic adverse effects. Therefore, the use of CHCs and DMPA should generally be avoided in patients with microvascular complications, vascular disease, or greater than 20 year duration of diabetes. An IUD, implant, or progestin only pill would be preferred for this population. Non-pharmacologic methods of contraception, like barrier methods including condoms, could also be considered for family planning, although the efficacy with typical use is less than the pharmacologic methods.7
Preconception counselling for patients with diabetes should include a discussion about the expected time to conception, and when to consider screening for infertility. DMPA users should be aware of a delayed return to fertility of up to 1 year after discontinuation, although this can vary for each patient.9 Females with diabetes may also struggle with infertility, and need specialist support.10 Polycystic ovarian syndrome shares a common pathology with Type 2 diabetes, so the two conditions often coexist.11 Prior to conception, optimising glycaemic control and obtaining and maintaining a healthy weight may improve fertility, but may not resolve all of the underlying causes.10
Although the focus of this review is primarily females with diabetes, males with diabetes who are planning for conception should also be counselled on the importance of controlling blood sugar to maintain fertility. A retrospective analysis of 110 males with diabetes referred to a urology clinic found that approximately 50% of male patients with diabetes presented with some degree of subfertility or infertility.12 Hyperglycaemia may be associated with erectile dysfunction, altered hormone levels, and decreased sperm function.13 Male patients with diabetes may need a referral to a urologist for evaluation of fertility concerns.14
WEIGHT MANAGEMENT
Achieving a healthy body weight prior to conception is recommended, as being overweight or underweight can be harmful to the developing fetus.15 Reproductive risks of obesity include infertility, miscarriage, birth defects, and preterm delivery.16 Low BMI during pregnancy has been associated with low birth weight.17 Medical nutritionists can provide individuals with counselling and lifestyle recommendations during the preconception period. Behavioural therapy to support lifestyle changes may be indicated. As much as is possible, medications for diabetes that are associated with weight gain (e.g., insulin, sulfonylureas, and thiazolidinediones) should be minimised, although intensification of previous pharmacotherapy may be needed to achieve goal HbA1c.18 The lifestyle recommendations mentioned in Table 1 help to support attaining a BMI between 20.0–24.9. For individuals needing to lose weight, it is recommended to maintain a 500 to 750 kilocalorie per day energy deficit.19
With increased public interest regarding medications such as semaglutide, dulaglutide, and tirzepatide, patients may enquire about these options for diabetes and weight loss. Because the full effects of these agents on the developing fetus are not yet known, it is recommended that glucagon-like peptide 1 agonists and tirzepatide be discontinued prior to conceiving.20 For patients in the preconception period who are on contraception, and not yet ready to conceive, it should be noted that tirzepatide is associated with reduced efficacy of oral hormonal contraception. Patients should be advised to use non-oral hormonal contraception or barrier contraceptive methods for 4 weeks after initiation of tirzepatide, and after each dose increase.21 Medications for weight loss are not recommended at conception.22
BLOOD GLUCOSE CONTROL
A taskforce convened by the International Endocrine Society published guidelines on treating diabetes and pregnancy in 2013. Based on observational research, suggesting that increased risk of fetal complications has been associated with HbA1c levels greater than 6.4%, they recommend that females with diabetes attempt to achieve blood glucose levels as close to normal as possible, while avoiding hypoglycaemia.23 Both the UK’s National Institute for Heath and Care Excellence (NICE) and the American Diabetes Association (ADA) recommend a goal HbA1c of less than 6.5% in the preconception phase to reduce risk of pre-eclampsia, preterm birth, macrosomia, congenital abnormalities, and complications of pregnancy.5,6 To decrease the risk of congenital abnormalities from hyperglycaemia, contraception can be prescribed until medications and HbA1c are optimised for pregnancy.5,6,15 Following conception, the goal HbA1c intensifies to less than 6.0%, as long as hypoglycaemia can be avoided.24-26 Because the rate of red blood cell turnover increases during pregnancy, the correlation between HbA1c and glucose levels changes. During pregnancy, the HbA1c will be lower relative to blood glucose levels, and blood glucose monitoring will be a more accurate measure of glycaemic control. The NICE guidelines for diabetes and pregnancy specifically recommend that females with HbA1c levels greater than 10.0% avoid trying to conceive until their HbA1c is lower, due to the associated risks.6 This recommendation is based on limited data that indicates a first trimester HbA1c greater than 10.0% is associated with congenital malformation rates as high as 20–30%.27,28
As access to continuous glucose monitoring (CGM) increases, more patients will have the ability to actively monitor their blood glucose, and avoid potentially harmful hypoglycaemia episodes. The CONCEPTT study evaluated use of CGM in pregnant patients with Type 1 diabetes, and found that use of CGM was associated with decreased HbA1c, increased time in glucose target, and decreased incidence of large birthweight infants.29 It is reasonable to extrapolate that use of CGM during the preconception period will benefit patients interested in this technology.
Pharmacotherapy for Blood Glucose Control
In females with Type 1 and 2 diabetes, once the decision has been taken to attempt pregnancy, guidelines recommend utilising insulin.5 For females with Type 1 diabetes, the majority are already solely on multidose insulin. If they have achieved tight control with their current insulin regimen, due to the risks of uncontrolled blood glucose and the limited differences among available insulins, they should keep it as is. For females with Type 2 diabetes, who may or may not be on insulin prior to pregnancy, insulin lispro, insulin aspart, and insulin detemir are preferred, due to having the most evidence with regards to safety.30 Although insulin regular and neutral protamine Hagedorn insulin are safe as far as congenital malformation risk, they are associated with a higher risk of hypoglycaemia in comparison to insulin analogues.30 Recent open label trials indicate that insulin degludec is safe and comparable to insulin detemir.31 Most of the data on insulin glargine are based on retrospective studies.32 Nonetheless, it is also considered safe. Metformin could be considered as an adjunct or alternative to insulin during preconception in patients with Type 2 diabetes.6 There are data to support its safe use in pregnancy, although insulin is still preferred.5 The long term effects of metformin use during pregnancy have not been established.30
Females who are on reliable contraception and regularly access healthcare may be on any available diabetes medications, and should be switched to insulin when a decision to attempt pregnancy is made. For females who are not planning pregnancy, but are on less reliable contraception, metformin is recommended as a first-line medication due to demonstrated safety during pregnancy.30 In patients on unreliable contraception, other first-line medications for diabetes (e.g., sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide 1 agonists) should generally not be withheld based on limited data in animals during pregnancy.33 The benefits of these medications to prevent maternal diabetes complications, such as atherosclerotic cardiovascular disease (ASCVD), outweigh the potential risk of early gestational exposure in an unplanned pregnancy. In this scenario, it is imperative that patients are counselled to contact providers if they believe they are pregnant, such that their medications can be changed as soon as possible.
Preconception Counselling Regarding Blood Glucose Management During Pregnancy
Although guidelines recommend insulin for preconception care, the transition from oral therapy to insulin can be difficult for many patients. As optimising glycaemic control during the preconception window is important in reducing fetal complications, clinicians should consider a patient specific approach when determining appropriate preconception therapy. Patients should be aware that insulin titration is a multistep process, and ideally should plan, so there is sufficient time to achieve glycaemic control prior to conception. Therefore, patients should be encouraged to discuss their current reproductive goals and desired timeline with their healthcare team at each visit.6 Patients should be encouraged to make their healthcare team aware of a pregnancy as soon as possible.
Additionally, during preconception counselling, providers should educate patients about the changes to blood glucose monitoring and insulin doses that are typically needed during pregnancy. Once pregnancy is achieved, quick dose adjustments and frequent monitoring to achieve glycaemic control will be required to prevent fetal harm and maternal complications.34 Insulin doses are anticipated to be increased throughout pregnancy, as insulin resistance is known to increase in the second and third trimesters.34 Patients should be counselled so they do not see this as their failure, but simply as an anticipated dose adjustment. If a patient is utilising metformin during the preconception period, or during pregnancy, they should be aware that in a large clinical trial investigating metformin use for gestational diabetes, metformin failed to achieve glycaemic control for 46% of patients, requiring a transition to insulin.35 This awareness is important, so that patients have realistic expectations, and can prepare themselves for a potential shift to insulin injections.
HYPERTENSION
Hypertensive disorders, including pre-existing hypertension, gestational hypertension (developing after 20 weeks gestation), and pre-eclampsia, are the primary cause of maternal morbidity and mortality worldwide.36
Pre-existing diabetes and chronic hypertension both increase the risk for developing pre-eclampsia.37 The American College of Obstetricians and Gynecologists (ACOG) recommends utilising a threshold of 140/90 mmHg to start antihypertensive therapy during pregnancy, rather than the previous recommendation of 160/110 mmHg.38 This is primarily based on the results of the CHAP trial, which demonstrated that targeting blood pressure less than 140/90 mmHg reduced the risk of severe pre-eclampsia and preterm birth.39 First-line medications used in the study included labetalol and extended-release nifedipine, with amlodipine and methyldopa used if preferred by the patient. The International Society for the Study of Hypertension in Pregnancy (ISSHP) recommends using antihypertensives to maintain blood pressure in the range of 110–140/80–85 mmHg during pregnancy.40 The Endocrine Society recommends achieving goal blood pressure of less than 130/80 mmHg prior trying to conceive.23
During preconception counselling, patients’ medication lists should be evaluated to identify any potentially harmful therapies, including angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), and spironolactone.5 These agents have been associated with complications, including fetal renal dysplasia, oligohydramnios, pulmonary hypoplasia, and intrauterine growth restriction. They should be stopped prior to conception, especially in patients not using reliable contraception. In those with persistent albuminuria or decreased estimated glomerular filtration rate, the risk of using an ACE inhibitor or ARB must be weighed carefully against the benefits of slowing progression to end-stage renal disease. As the pre-pregnancy severity of chronic kidney disease (CKD) increases, the risk of pregnancy-related complications increases, as well as worsening of the CKD itself during pregnancy.41 Therefore, it is reasonable to use an ACE inhibitor or ARB in a patient with CKD, as long as they are on reliable contraception. The adverse effects associated with these medications occur primarily during the second and third trimesters; the ACE inhibitor and ARB should be stopped once a decision to attempt pregnancy is made, or pregnancy has occurred.42 Acceptable pharmacologic agents for pregnant individuals include labetalol, methyldopa, nifedipine, and diltiazem.40 Prazosin and hydralazine may be used as second- or third-line agents. Home blood pressure monitoring is useful to encourage patient involvement in care, as well as for screening for white coat hypertension. Non-pharmacologic therapy to reduce blood pressure includes all lifestyle recommendations in Table 1, as well as restricting dietary sodium to less than 2.3 g per day.43
DYSLIPIDAEMIA
Patients with diabetes should be screened for dyslipidaemia during an initial preconception health assessment.5 The majority of individuals who are pregnant should not be on lipid-lowering treatment during pregnancy. Selected individuals with familial hypercholesterolaemia or pre-existing ASCVD may require treatment.44 There is controversy regarding the teratogenicity of statins. There has been a long-held belief that statins cause fetal abnormalities, and therefore would be contraindicated in pregnancy; for this reason, bile acid sequestrants were used, despite limited data indicating ASCVD protection. Observational data do not support this finding, and multiple large-scale studies have demonstrated no increased risk of fetal abnormalities seen in patients who became pregnant while on a statin.45 These data are helpful in reassuring patients who may experience an unintended pregnancy while on a statin. However, observational data are not enough to rule out all fetal harm, and the increased risk of spontaneous pregnancy loss. A study found a correlation with statin use and an increased risk of miscarriage in patients using statins 3 months before conception, or during pregnancy.46 Therefore, it is still not recommended to use statins during preconception or pregnancy in most patients. There may be a small subset of patients where the benefits of statin treatment, including reduction in mortality for those at very high-risk of an ASCVD event, may outweigh the potential risk of harm during pregnancy.45 The ADA and NICE guidelines recommend discontinuing statins prior to conception, and avoiding their use in patients with childbearing potential that are not on a reliable form of contraception.5,6
DIABETES COMPLICATIONS
All individuals with diabetes should be regularly evaluated for diabetes complications, such as retinopathy, neuropathy, nephropathy, and ASCVD. During preconception planning, these evaluations are even more important, since they potentially worsen during pregnancy, and can increase maternal morbidity. For example, individuals with proliferative retinopathy should be treated with laser photocoagulation surgery prior to conception to minimise worsening.47 Anti-vascular endothelial growth factor medications can be used preconception, but should not be used during pregnancy, due to concerns regarding impact on the blood vessels of the fetus.47 Also, in individuals with retinopathy, blood glucose lowering should be gradual, since rapid lowering is associated with further damage to the ocular blood vessels.47 Gastrointestinal neuropathy can affect nutrition, and is associated with extreme fluctuations in blood glucose, increased risk of diabetic ketoacidosis, and hospitalisations.48 Diabetic peripheral neuropathy remains stable in most females during pregnancy, but medications used to treat neuropathic pain may be harmful to the fetus. Topical treatments like capsaicin are the safest medications to use for treatment of neuropathy during pregnancy, and if oral treatment is needed, gabapentin, based on limited data, is likely safe, although it does cross the placenta.33
Renal insufficiency generally does not progress during pregnancy, but is correlated with an increased risk of pregnancy complications, such as pre-eclampsia and premature delivery.49 A history of ASCVD during pregnancy is associated with increased maternal mortality; females who are older than age 35, with evidence of microvascular disease or with signs of possible cardiovascular disease, should be screened with an electrocardiogram.49
CONCLUSION
Despite the lack of a cure for diabetes at this time, the treatment of diabetes has progressed tremendously over the past decade, with greater knowledge, technology, and medication options, allowing for fewer patients who experience diabetes complications. Tables 2 and 3 summarise the recommendations set forth in this review for patients in different stages of preconception. With greater numbers of individuals in their childbearing years with diabetes, the benefits of the treatments must be weighed carefully against the potential harms to a fetus. The guidelines are clearer when the preconception timeframe is short, and pregnancy is planned. In these circumstances, patients can be switched to safer blood pressure medications, medications for dyslipidaemia can be temporarily stopped, and insulin can be initiated if pharmacotherapy is needed to reach blood glucose goals. The more challenging situation is when the preconception period may be longer than anticipated, in which case, the risk of diabetes complications must be considered, since maternal diabetes complications increase pregnancy-related maternal and fetal morbidity and mortality. For patients with Type 2 diabetes, reducing diabetes complications, reaching blood glucose goals, and achieving a healthy weight may be easier to accomplish with newer medications, for which we have less information regarding safety during pregnancy.
Ultimately, healthcare professionals are guides for our patients in helping them to reach their health-related goals. When providing guidance, patient-specific factors must be taken into consideration. These factors include financial considerations; housing circumstances, like access to refrigeration to store insulin; and patient comfort with intensifications of therapy. Educating patients in a way that is empathetic and clear will help individuals with diabetes feel empowered to ask questions, and take ownership of their health during preconception.
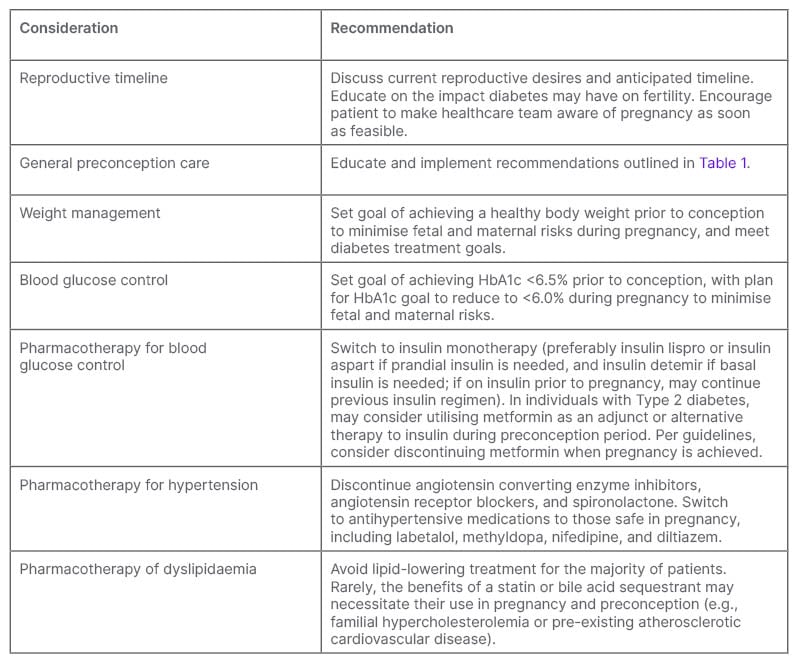
Table 2: Preconception care for females with pre-existing diabetes attempting to become pregnant, or planning pregnancy in the imminent future.
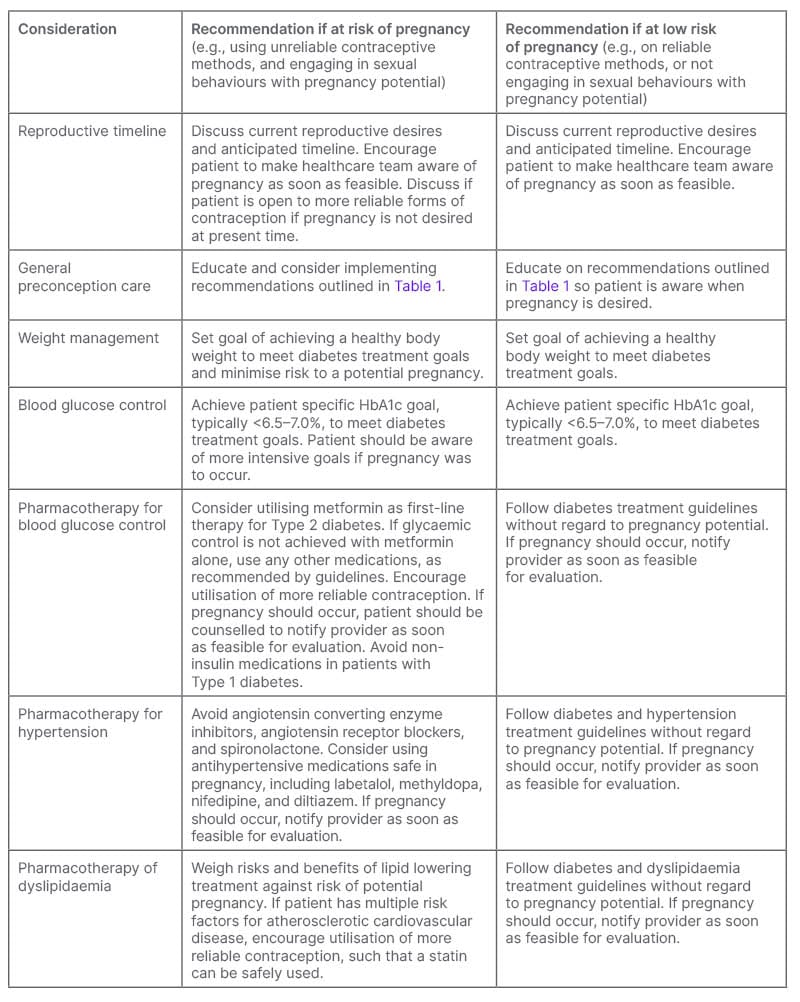
Table 3: Preconception care of females with pre-existing diabetes not currently planning for pregnancy.







