Abstract
The twin epidemics of Type 2 diabetes (T2D) and obesity will continue to bring significant health challenges in the coming decades. Randomised controlled trials of glucagon-like peptide 1 (GLP-1)-based therapies showed high glycaemic efficacy with clinically meaningful weight loss, and have been considered as game-changers in the diabesity population. Emerging evidence has demonstrated that co-administration of glucose-dependent insulinotropic peptide (GIP) and GLP-1 results in enhanced insulinotropic effect in an additive way with significant glucagonostatic response, compared with the administration of each hormone separately. These findings have driven the choice to pursue incretin-based dual agonist therapies, known as ‘twincretin’. Observations from the global registration Phase III trials suggest that tirzepatide (a novel dual GIP/GLP-1 receptor agonist) represent advancement over current GLP-1 analogues, providing enhanced glycaemic and weight benefits with similar gastrointestinal tolerability. However, data are limited from patients with a range of ethnicities, and several questions remain unanswered.
Key Points
1. Co-administration of GIP and GLP-1 results can produce an enhanced insulinotropic effect compared to the administration of each hormone separately.2. Results on the long term efficacy, safety, and tolerability, from ongoing Phase III clinical trials will impact the therapeutic prospects and future directions of incretin-based agonist therapy.
3. Incretin-based dual agonist therapy may offer an alternative to stringent dietary restriction and challenge the success of bariatric surgery in the diabesity community
INTRODUCTION
The incretin effect, an augmented prandial insulin response, is mediated mostly by the hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP), which are released from the intestines after meals.1 This effect is part of the entero-insular axis of glucose homeostasis, and is responsible for 50–65% of total insulin secretion after a meal.2 The first incretin described in 1973 was GIP, and GLP-1, the second incretin, was discovered later in 1987.3,4 GLP-1 and GIP are the two most widely studied incretins. GIP perhaps makes a greater contribution to the physiological insulin response to meals than GLP-1, but therapeutic development to date has focused on GLP-1, as the insulinotropic effect of GIP is markedly attenuated in Type 2 diabetes (T2D).5 GLP-1 receptor agonists (GLP-1RAs) are established glucose-lowering agents that potentiate insulin secretion in response to glucose, and suppress glucagon secretion. GLP-1RAs are associated with greatest weight loss, and have shown their ability to decrease the risk of cardiovascular (CV) events in individuals with T2D at risk of CV disease.6 Nevertheless, renewed interest in the therapeutic potential of GIP emerged, with studies showing that improving glycaemic control with another agent such as insulin could restore the insulinotropic potency of GIP.7,8 The novel concept of combining a GIP with a GLP-1RA takes incretin-based therapy to a new level. This review will explore whether complementary mechanisms of action of combined GLP-1 and GIP receptor agonism could be effective and safe new options that are superior to pure GLP-1RAs as therapy for metabolic disorders.
GIP PHYSIOLOGY: SIMILARITIES AND DIFFERENCES WITH GLP-1
GIP is a 42-amino acid peptide secreted from intestinal K cells that are located mainly in the proximal small intestine. Like GLP-1, GIP is released after ingestion of nutrients, and promotes meal-stimulated insulin secretion in a glucose-dependent manner by receptor binding to pancreatic β-cells.9 However, GIP has a distinctly modulatory effect on glucagon by inhibiting its secretion instates of hyperglycaemia, but increasing glucagon release during hypoglycaemia. The half-life of GIP and bioactive GLP-1 is only a few minutes because they are inactivated by the enzyme dipeptidyl peptidase 4.10 GIP also targets bone, acutely inhibiting bone resorption.11 Unlike GLP-1, GIP exerts anabolic actions at the level of adipose tissue leading to accumulation of body fat.12 The effect of GIP on the gastrointestinal (GI) tract is incompletely understood; while GLP-1 slows gastric emptying,13 this effect is not confirmed for GIP.
Clinical studies exploring the effect of GIP on food intake are limited. However, GIP appears to synergise the central satiety effect and weight loss linked with GLP-1. GIP might also improve cognitive function and certain aspects of lipid profile, but its CV effects are not yet fully elucidated. The superior metabolic benefits observed with concomitantly targeting GLP-1 and GIP receptors garnered interest in developing unimolecular drugs that would combine the actions of GLP-1 and GIP (Figure 1).
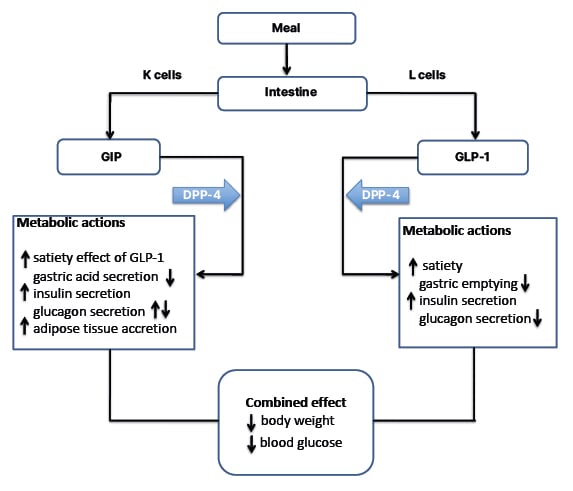
Figure 1: Metabolic actions of GLP-1 and GIP on key target tissues.
Upward arrows denote an increase, downward arrows denote a decrease.
GIP: glucose-dependent insulinotropic peptide; GLP: glucagon-like peptide 1.
CONCEPT OF TWINCRETINS IN MANAGEMENT OF TYPE 2 DIABETES
It has been postulated that the glucose-lowering effects of simultaneous GLP-1R agonism might help overcome resistance to GIP action in T2D, thus enabling greater glucose-lowering effects when combined. In principle, treatment with a GIP and a GLP-1 RA together would augment glucose lowering while providing defence against hypoglycaemia. Besides, GLP-1 and GIP appear to activate distinct hypothalamic mechanisms coupled to reduced food intake,14 providing a greater degree of weight lowering than that seen with GLP-1 alone. Although pharmacological levels of GIP can increase glucagon secretion and promote adipose tissue deposition, these effects can be countered by inhibition of glucagon secretion and reduction of food intake linked with GLP-1.15 Altogether, this has brought the novel concept of incretin-based dual agonist therapy.
The first ‘twincretin’ was a unimolecular dual GLP-1R/GIP receptor (GIPR) agonist. It demonstrated superior dose-dependent weight loss and reductions in blood glucose levels, with decreased food intake relative to exendin or liraglutide in rodents and monkeys.16 The leading agent among unimolecular GLP-1/GIP co-agonists is tirzepatide, a 39 amino acid synthetic peptide with agonist activity at both the GIP and GLP-1 receptors, with a higher affinity to GIPR.17 Its structure, which is primarily GIP sequence-based, includes a C20 fatty di-acid acyl chain that extends the duration of action, thereby allowing once-weekly subcutaneous administration. Initial clinical evaluation of tirzepatide included a series of randomised, placebo-controlled Phase I trials designed to assess the safety, tolerability, pharmacodynamics, and pharmacokinetics of the molecule in humans.17 In Phase II studies, weekly doses of tirzepatide ranging up to 15 mg, resulted in up to 2.4% reductions in HbA1c and weight loss of up to 11.3 kg in individuals with T2D.18 Tirzepatide induced greater improvements compared with dulaglutide in a study that measured different markers of β-cell function and insulin sensitivity.19 It was also associated with improvements in lipoprotein profiles.20 Tirzepatide is the first twincretin to enter global Phase III trials.
TIRZEPATIDE: EFFICACY IN PHASE III CLINICAL TRIALS
After the promising results in Phase I and Phase II trials, the SURPASS clinical trial programs were designed to assess the efficacy and safety of Tirzepatide as a treatment to improve glycaemic control in patients with T2D. The SURPASS Phase III clinical trials (Table 1)21-29 are mostly multicentric and global; only two studies are from Japan and one study is from the Asia–Pacific region. These trials included patients with different background therapies; for example, SURPASS 1 included treatment of naïve patients, whereas others included patients on different oral anti-diabetic agents (metformin, sulfonylurea, pioglitazone, SGLT-2 inhibitor, and/or insulin). SURPASS 1, SURPASS 5, and SURPASS J-combo are placebo controlled, while others have active comparators: for example GLP-1RAs (dulaglutide and semaglutide), long-acting insulin analogues (glargine and degludec), or short-acting insulin analogue (lispro). The SURPASS trials are designed to evaluate once-weekly tirzepatide doses of 5 mg, 10 mg, and 15 mg with a dose escalation algorithm: starting dose at 2.5 mg weekly for the first 4 weeks, then dose increment of 2.5 mg every 4 weeks until the maintenance dose of 5 mg, 10 mg, or 15 mg is achieved. Other than the SURPASS J-combo trial, the primary endpoint for each of the studies was the change in HbA1c from baseline. To date, the results of SURPASS 1–5 have been available, and it has been shown that tirzepatide lowers HbA1c significantly better than placebo, semaglutide, degludec, glargine, and placebo, respectively. In addition, there was significant weight loss seen as compared with placebo, insulin, or semaglutide. The SURPASS 2 trial was a head-to-head comparison with one of the most effective GLP-1 analogues, semaglutide, in patients only on metformin. This trial showed noninferiority and superiority to semaglutide for HbA1c reduction, as well as for weight reduction. In a recent meta-analysis which included data from six randomised control trials involving 3,484 patients, tirzepatide showed impressive glycaemic efficacy and weight loss data over 1 year of use.30 The results of SURPASS J-combo, SURPASS AP, and SURPASS 6 are awaited.
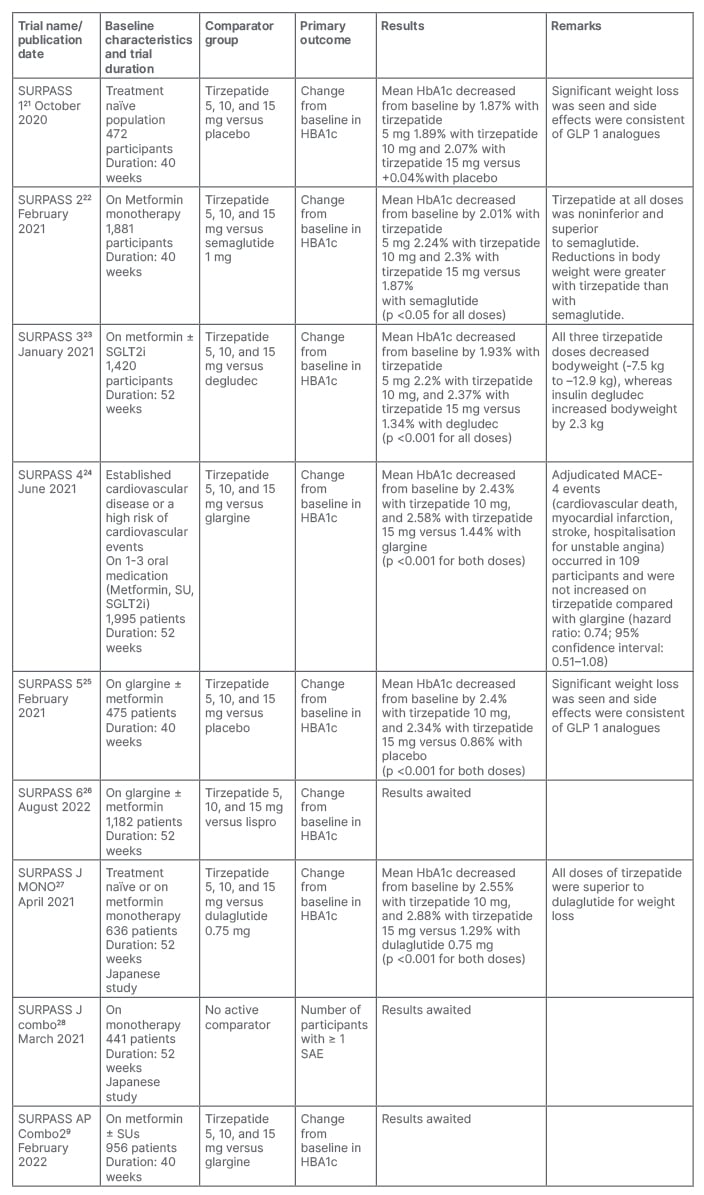
Table 1: Overview of the SURPASS Phase III clinical trials of tirzepatide for the treatment of Type 2 diabetes.
SAE: serious adverse effects; SU: sulfonylurea
SAFETY AND TOLERABILITY OF TIRZEPATIDE
Overall, the safety profile is at par with the existing GLP-1RAs. The most common side effects reported in SURPASS 1 trial (as compared with placebo) were abdominal discomfort, nausea, diarrhoea (12–18%), injection site reactions (2–3%), and hypersensitivity (1–2%); however, all side effects were mild. Although there was rise in the amylase level with tirzepatide treatment compared with placebo, there was no confirmed case of pancreatitis. Furthermore, no treatment emergent diabetic retinopathy or medullary carcinoma of the thyroid was reported. When compared head-to-head with semaglutide in the SURPASS 2 trial, nausea was reported in 17–22% of patients who received tirzepatide, and in 18% who received semaglutide. Diarrhoea was reported in 13–16% and 12%, respectively, and vomiting in 6–10% and 8%, respectively. This indicates that the GI side effects of tirzepatide and semaglutide are comparable. Pancreatitis and cholelithiasis were reported in small number of patients (<1%), and were similar in both groups. When compared with degludec in the SURPASS 3 trial, GI side effects were more common in the tirzepatide group, but hypoglycaemia was more common in people treated with degludec (1–2% versus 7%). Although the dedicated CV outcome trial (CVOT) is still underway, adjudicated 4-P MACE was evaluated in the SURPASS 4 trial, and there was no increase in CV death, myocardial infarction, stroke, or hospitalisation for unstable angina in the tirzepatide group as compared with glargine (hazard ratio: 0.74; 95% confidence interval: 0·51–1·08).25 Satter et al.31 demonstrated similar safety in 4-P MACE in a meta-analysis which included 4,487 participants treated with tirzepatide and 2,328 participants in control arm from seven trials which lasted more than 26 weeks duration.
THERAPEUTIC PROSPECTS
To date, the efficacy and safety trials of tirzepatide are mostly published. However, dedicated data from Japan and the Asia Pacific region (SURPASS J-combo and SURPASS AP-combo) are yet to come. The results of the ongoing SURPASS-CVOT trial will be published in 2024 (Table 2).32-37 This large trial is likely to provide more clarity on whether dual agonism through tirzepatide lead to even greater benefits in patients with T2D and established CV disease than a GLP-1RA based approach. As there was clear evidence of clinically significant weight loss from the Phase III trials, tirzepatide has been planned to be evaluated as an anti-obesity drug in non-diabetic (SURMOUNT 1 trial), and diabetic (SURMOUNT 2 trial) populations, and on those who are already on intensive lifestyle programmes (SURMOUNT 3 trial). Of these, the SURMOUNT 1 trial33 has recently been published, which found significant weight loss with all three doses of tirzepatide compared with the placebo in non-diabetic individuals. This indicates tirzepatide may be a potential therapeutic option for individuals living with obesity. Another two trials are currently ongoing. The SUMMIT trial will document safety and efficacy of tirzepatide in heart failure patients, and the SYNERGY-NASH trial will provide specific data on the benefit of treatment with tirzepatide in people with non-alcoholic steatohepatitis. If the results of these trials are positive, it will open a new horizon in the management of T2DM, obesity and non-alcoholic steatohepatitis.
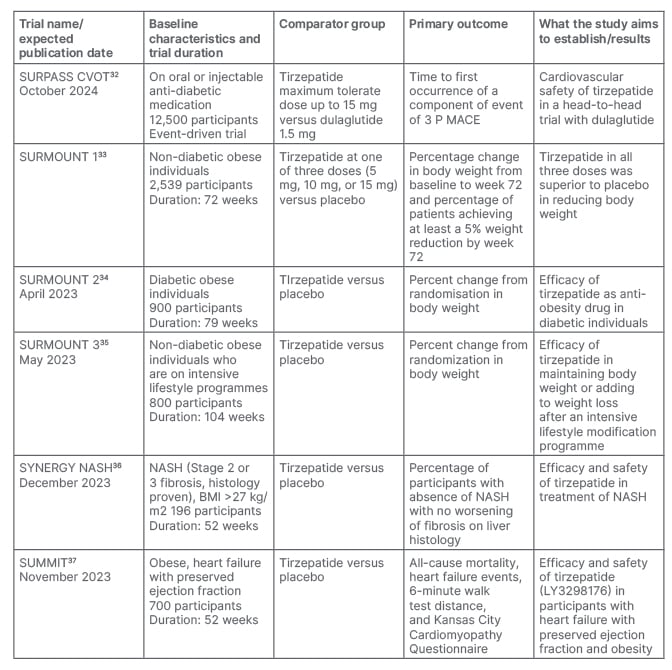
Table 2: Published and ongoing trials on tirzepatide for cardiovascular safety, obesity, and non-alcoholic fatty liver disease.
NASH: non-alcoholic steatohepatitis
OUTSTANDING QUESTIONS AND FUTURE DIRECTIVES
Although there is a lot of evidence at present with this novel molecule, many unanswered questions remain. Firstly, it is not clear how much GIPR stimulation contributes to the effects of tirzepatide, and what should be the ideal ratio of GIP/GLP-1 action of this co-agonist for the best results. Secondly, do these unimolecular agents target both receptors in the same cell, different cells in different tissues, or both? Thirdly, the action of GIP on β-cells has a tachyphylaxis effect; what will be the effect of tirzepatide in this regard? Furthermore, a layer of complexity underlying the logical development of GIP/GLP-1R co-agonists grow from numerous studies, indicating that GIPR antagonism may be metabolically beneficial as well. Nevertheless, GIPR antagonist is now available on the market with promising initial results, and can be utilised to understand the clinical benefits seen with tirzepatide. Lastly, the future positioning of dual GIP/GLP-1RA against GLP-1RA in the treatment landscape of T2D needs to be better defined. The positioning of tirzepatide in the therapeutic algorithm will likely be influenced by emerging informations on CV outcomes, non-alcoholic fatty liver disease, kidney protection, and durability of effects. One promising aspect of incretin-based dual agonist therapy (e.g., tirzepatide) is that it might offer an alternative to stringent dietary restriction, and could challenge the success of bariatric surgery in the diabesity community.







