Abstract
![]()
Erratum: Newer Oral Levothyroxine Formulations: Is It Time to Switch Over?
Author: Venkatraman Rajkumar
Original citation: EMJ. 2023; DOI/10.33590/emj/10306765. https://doi.org/10.33590/emj/10306765.
Date correction published: 15.03.23
The article by Rajkumar was originally published on 24.10.22. Since then, an erratum has been made. In Figure 1, “hypothyroidism” was misspelt as “hyptothyroidism”. This has now been rectified. Also in Figure 1, an asterisk was missing, which meant that there was information in the legend that is not linked to the figure. This has now been updated.
EMJ apologises for the error and any inconvenience caused.
![]()
INTRODUCTION
Hypothyroidism is a common condition encountered in primary care and is prevalent worldwide. Primary hypothyroidism due to autoimmune thyroid disease is the most common aetiology, followed by thyroidectomy and radioiodine therapy. Secondary and tertiary hypothyroidism due to pituitary and hypothalamic diseases, respectively, are rare in primary care. Hence, only primary hypothyroidism is discussed. Thyroid hormone replacement therapy is the treatment for hypothyroidism of any cause. In the remote past, preparations extracted from dried animal thyroid were used. Subsequently, the categorisation of the hypothalamic-pituitary-thyroid axis, the discovery of thyroid hormones, their structure, kinetics, and feedback regulation led to levothyroxine (LT4) as the standard replacement therapy. A newly diagnosed patient is started on therapy given as a tablet once daily in the morning. A thyroid stimulating hormone (TSH) level in the normal range is the goal of therapy which takes 6–8 weeks to attain when adequately dosed. A log-linear relationship exists between TSH and free T4 (FT4). For small changes in FT4, there is a large inverse shift in TSH, qualifying the latter as a more sensitive test for diagnosis and follow-up.1,2
Natural Thyroid, Levothyroxine, and Combination Therapy
The normal thyroid secretes both thyroxine (T4) and triiodothyronine (T3) in a ratio of 13:1. T4 acts as a pro-hormone and is deiodinated to T3 in peripheral tissues like the liver. Most of the T3 in the body (around 80%) is obtained from peripheral deiodination of T4. The latter is the effector hormone in all tissues. It is this fact that the proponents use for advocating T4 monotherapy.3 Although suitable for most patients, some 10–15% continue to suffer symptoms and a poor quality of life, despite achieving therapeutic goals. Opponents of sole therapy with LT4 have advocated more physiological therapy using a combination of T4 and T3. While natural thyroid preparations contain both T4 and T3, these animal products have a much higher T3 content (T4 to T3 ratio of 4:1). Moreover, there is variation in the contents between batches that provide erratic levels, leading to suboptimal care.3 This therapy is, therefore, not recommended by experts.
Studies using a combination of T4 and T3 have failed to prove superiority over T4 monotherapy.4 The T3 used in these studies was short-acting, and dosed one to three times daily. This produces high levels shortly after dosing, and low trough levels before the next dose. This fact is used for refuting these studies. To mimic the bodily kinetics, a long-acting T3 is a solution. The Phase I trials with long acting T3 (T3 LA) have shown some promise.3,5 Until definite results are obtained from more robust trials with a combination of T4 and T3 LA, LT4 will remain the standard of care for hypothyroidism.
Depending on the clinical scenario, LT4 is used by oral, intravenous, intramuscular, subcutaneous, or rectal routes. Nasal spray formulations are being studied.6 Although the other routes are useful for certain clinical situations, such as with patients who are critically ill, oral LT4 in tablet form (LT4tab) is the choice for patients who present to primary care.7
Levothyroxine Preparation and Kinetics
LT4 itself is very unstable and rapidly degraded in the milieu of the stomach. Treatment with alkali in the manufacturing laboratory yields LT4 sodium, which is more stable in the gastric environment. After ingestion, the stomach acid helps disintegrate the LT4tab. An optimal pH of the gastric contents delivered to the duodenum is essential for proper absorption in the duodenum, jejunum, and ileum via specific transporters in the intestinal epithelial cells. The absorbed LT4 reaches the liver. Here, apart from deiodination to LT3, some of the LT4 gets converted to sulfates and glucuronides via alternative routes of metabolism. Some of these metabolites reach the gut via biliary excretion. Here, they are converted to free T4 by intestinal bacteria and reabsorbed to complete the enterohepatic cycle. Between 60–80% of an oral dose of LT4tab is bioavailable. Given the complexities involved in the kinetics of LT4tab, many factors can interfere with its smooth absorption (Table 1). The consequences are frequent laboratory and physician visits that result in constant dosage changes, increased expense, and suboptimal care. The development and testing of novel oral formulations raise hope for a solution to this problem.8
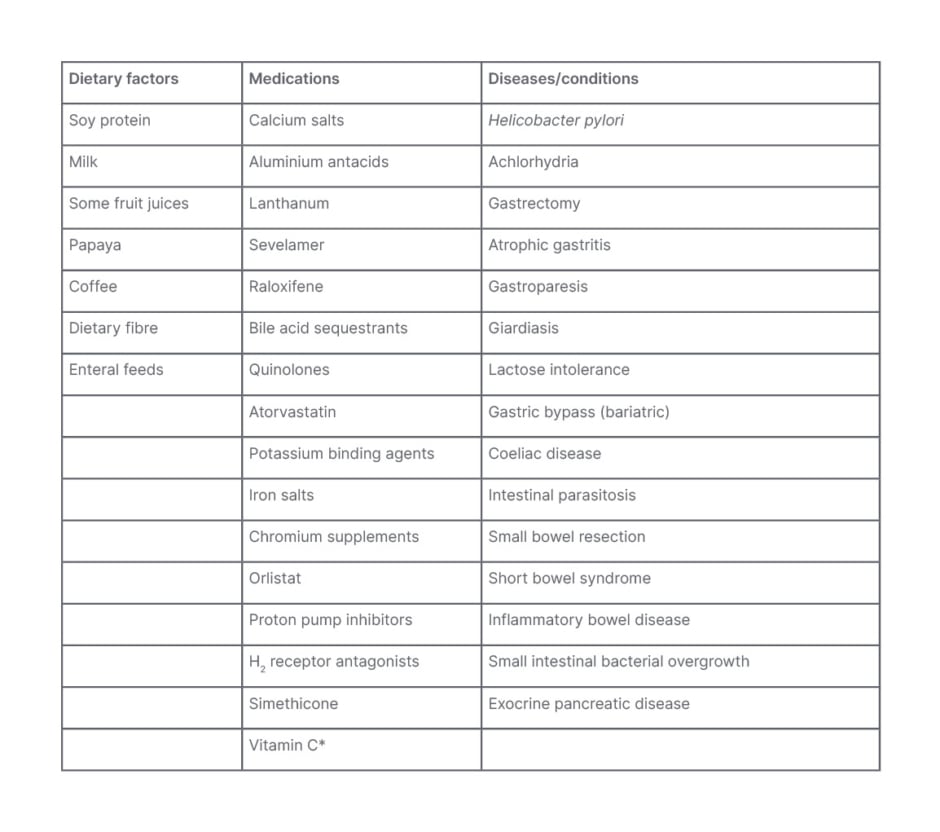
Table 1: Factors affecting absorption of oral levothyroxine.
*Increases levothyroxine absorption.
What is in an levothyroxine tablet?
Apart from LT4 sodium, each tablet contains many inactive ingredients. These include binding agents, preservatives, and permitted colouring agents, among others. They serve various functions, including binding the active ingredient, improving stability, and facilitating disintegration, dissolution, and dispersion. There are variations in the type and amount of these excipients that may explain some differences in bioequivalence between different brands and generics. Manufacturers are constantly trying to improve the quality of their products by manipulating these substances.7 Table 2 compares characteristics of levothyroxine tablet with the newer formulations.
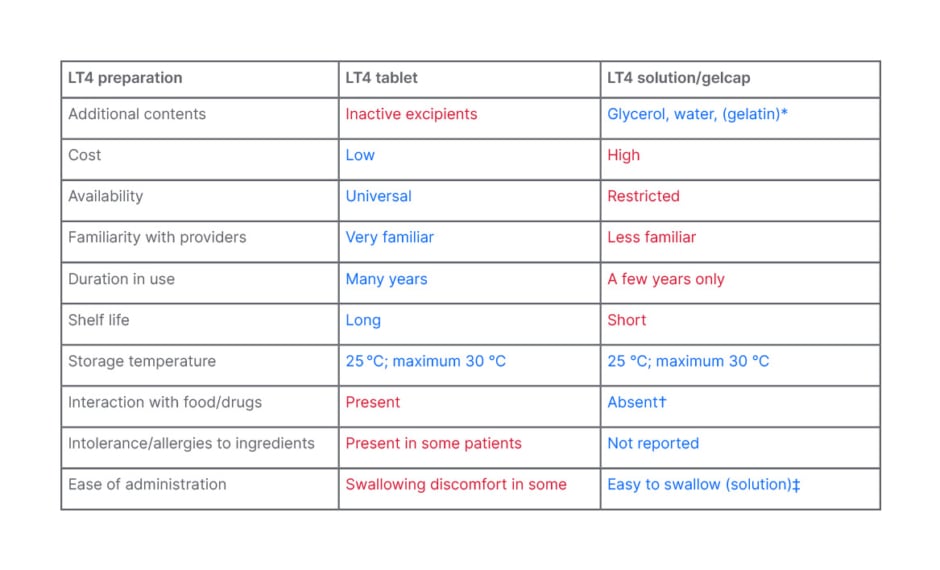
Table 2: Comparison of levothyroxine tablet and oral solution/soft gel capsule.
Blue depicts advantages.
Red depicts disadvantages.
*Gelatin outer shell in soft gel capsule dissolves and releases liquid levothyroxine.
†Claimed by studies; however, product leaflet suggests spacing with food and medications.
‡Soft gel cap needs to be swallowed, and may cause discomfort in some susceptible patients.
LT4: levothyroxine
In the quest for a solution to the problems with LT4tab described, investigators have ventured into levothyroxine solution (LT4sol) and levothyroxine soft gel capsules (LT4gelcap) as suitable alternatives.8,9
What is in the levothyroxine solution and levothyroxine soft gel capsules?
LT4 sodium, glycerol, and water are the constituents. Some previous LT4sol preparations included alcohol or propylene glycol in small quantities. In the LT4gelcap, a gelatin outer covering is present in addition, which quickly dissolves in the stomach.10
Bioequivalence, a prerequisite for new formulations
The U.S. Food and Drug Administration (FDA) and other regulatory agencies have mandated bioequivalence tests before approval of new drugs. For LT4, the area under the curve and maximal concentration achieved, measured as the FT4 concentration after administering 600 mcg as a single dose in the fasting state, should be close to that of a standard LT4 preparation. Initial tests performed showed that the newer compounds achieved bioequivalence to standard LT4tab.11 The FDA approved the LT4sol in 2017, whereas it was approved earlier in Europe. Yue et al.9 studied the kinetics of LT4sol and showed the earlier time to maximum concentration and greater area under the curve for LT4sol. This fact alone may be the main contributor to its advantages when administered with food or medications.9 It is not surprising that the LT4tab needs time to dissolve and attains blood levels later. Yamamoto12 reported three patients in whom powdered LT4 tablets solved the absorption issues with the whole tablet. If similar results are obtained in many different clinical situations in a larger population, it could be a much cheaper alternative to the much-touted and more expensive LT4sol.
EVIDENCE IN LITERATURE
Summary of Studies with Concomitant Food, Medications, or Conditions
There are numerous case reports involving a single or a small number of subjects. Many examined the effects of food, concomitant medications, gastrointestinal disease states, bariatric surgery, radioiodine thyroid ablation, and thyroidectomy. Most results rated LT4sol as superior or equivalent and none inferior to LT4tab. All studies had one or more deficiencies: insufficient power (small number of subjects), not blinded, unmatched, insufficient washout periods, retrospective and non-uniform design, and a strong component of compliance bias in favour of LT4sol during the crossover.13,14 Among the various studies, the TICO study was prospective, placebo-controlled, double-blind, and well designed. It showed that LT4sol was not influenced by both food and medications. The drawbacks of this study were that it included subjects from a single country partaking a standard diet, and the absence of a comparative LT4tab arm to prove its superiority over the latter in a head-to-head comparison.15
Studies Addressing Temperature Stability and Storage Issues
Boulton et al.16 studied the effects of temperature and preservative on liquid thyroxine prepared by compounding crushed tablets. They concluded that a prepared LT4sol was least stable with added preservative and most stable at 4 ℃ with no added preservative.16 Benvenga et al.17 described the instability of LT4tab when stored in less than optimal conditions (proximity to room heaters, clear bottles, and transfer from blister packs into alternate storage). Subjects who took these poorly-stored pills reported abnormally high TSH levels. Subsequently, when these patients took properly stored pills, the TSH returned to normal.17
Bernareggi et al.18 added LT4sol to breakfast beverages, warmed them, and analysed LT4 levels in these foods by reliable methods. They showed good retrieval, even after subjecting the LT4sol to temperatures as high as 50 ℃.18 While this may prove convincing, no one is going to add LT4 in whatever form into their foods and heat it before consumption, nor is a temperature of 50 ℃ ever attained in the body. A better alternative is to test whether LT4sol is stable in the slightly higher temperatures that occur in the milieu of the tropical pharmacy for its entire shelf life. Most drug manufacturers recommend a storage temperature of 25 ℃ or less. It is the room temperature in most temperate climates. In warm climates like tropical countries, the average ambient temperature in most pharmacies is 30 ℃ or more. They invariably have no air conditioners, only ceiling fans. The LT4tab stored in these ‘hot’ areas has withstood high temperatures and generally seems effective given the on-target TSH levels with the usual replacement doses in most patients (Rajkumar, unpublished data). There are very few ‘real-world’ studies on the effects of sunlight, artificial room light, and temperature on levothyroxine tablets.19,20 These studies suggested that sunlight exposure, but not room light or heat exposure, degrades the drug. This corroborates the author’s observations on heat exposure. A careful reading of the prescribing information of a standard brand of LT4tab gives the answer. In the product insert, the upper limit of the storage temperature is stretchable to 30 ℃. It begs the question: how stable is LT4sol in higher temperatures, and for how long? The LT4sol supplied in single-dose ampoules is not stable beyond 2 weeks after opening. The package insert also cautions on spacing with food and medications.21
CONCLUSION
The most common reason for poor control is non-compliance. The patient, caregiver, and practitioner should partner in ensuring compliance. There may be a role for LT4sol in selected patients to justify the cost. Figure 1 outlines the approach to ‘resistant’ hypothyroidism proposed by the author. This approach is line with the expert consensus panel from Europe.22 A good history and evaluation for correctable causes should be undertaken. However, there will remain a handful of patients where a levothyroxine absorption test may be necessary. Here the art of convincing the insurers will win over clinical competence.
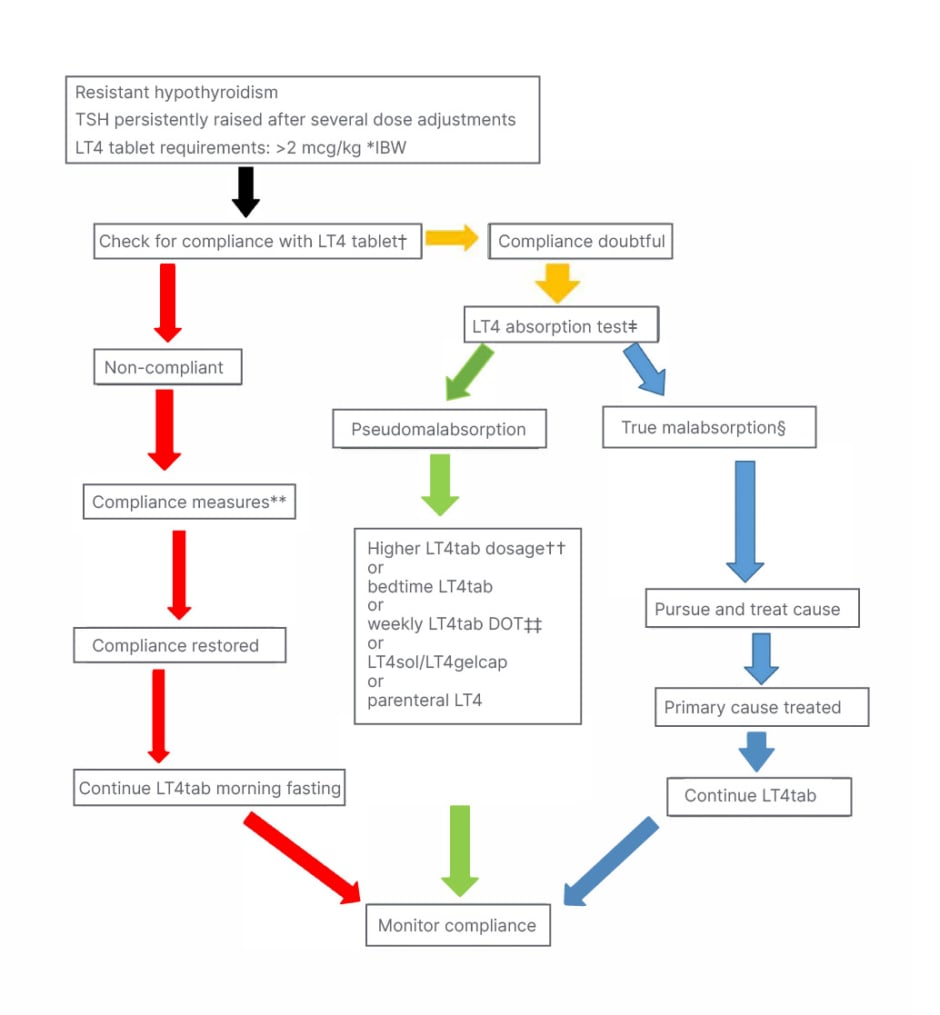
Figure 1: Author’s approach to resistant hypothyroidism.
*Correlates better than actual body weight for computing LT4 dose.
†Ask open-ended non-judgemental questions, interview caregivers, and check mood and cognition. ‡Many versions available; consult Endocrinology.
§May need alternative modalities of LT4 until primary malabsorption resolves.
**Use education tools tailored to literacy; use all available resources.
††Any one of the modalities may work; personalised approach needed.
‡‡Total weekly LT4tab requirements as single weekly dose.
DOT: directly observed therapy; IBW: ideal body weight; LT4: levothyroxine; LT4gelcap: levothyroxine soft gel capsules; LT4sol: levothyroxine solution; LT4tab: levothyroxine tablet.
A large, double-blind, multicentre, prospective, randomised, crossover trial comparing LT4tab with LT4sol will answer many burning questions. There is a need to represent paediatric and geriatric groups, all races, and geographical regions in these studies. The difficulty in blinding because one product is a tablet and the other is a solution is solved by utilising a double-dummy model: a placebo tablet that looks exactly like the LT4tab given with LT4sol, and a placebo solution that looks, feels, and tastes like the LT4sol used with the LT4tab for the study. After an adequate washout period, a crossover can be done, and an analysis performed.
Presently, there is insufficient evidence to justify a switch to LT4sol from LT4tab for millions of patients with hypothyroidism. The results of studies with the LT4sol, LT4gelcap, combination therapy of LT4 with LT3 LA, and nasal spray, will decide the future of LT4 replacement therapy. Like all new medications, the prices of LT4sol may become competitive in the future. At that time, LT4tab and LT4sol may become interchangeable. Recombinant human insulin has practically replaced animal-sourced insulin. LT4sol has to wait, at least for now.







