Abstract
Type 2 diabetes mellitus is currently the main cause of chronic kidney disease, leading to end-stage renal disease in most countries around the world. Metformin is the most commonly prescribed oral antihyperglycaemic in the world and after approval by the U.S. Food and Drug Administration (FDA) in 1994, it is currently recommended as the first-line pharmacological agent for newly diagnosed Type 2 diabetes mellitus by many professional diabetes associations. In this review, the authors analysed efficacy and safety of metformin in patients with chronic kidney disease.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is currently the main cause of chronic kidney disease (CKD) leading to end-stage renal disease (ESRD) in most countries around the world. Over the past decade, the United States Renal Data System (USRDS) figures have demonstrated a progressive increase in the number of T2DM cases entering ESRD programmes.1 Now, more than 40% of all incident patients are diabetic, while other classic nephrology disorders, such as glomerulonephritis, cystic kidney disease, and hypertension, have remained relatively steady as causes of ESRD over the past decade.2
Metformin is the most commonly prescribed oral antihyperglycaemic in the world and, after approval by the U.S. Food and Drug Administration (FDA) in 1994, is currently recommended as the first-line pharmacological agent for newly diagnosed T2DM by the American Diabetes Association (ADA)3 and by the European Association for the Study of Diabetes (EASD).4 It acts as an antidiabetic drug via increasing peripheral glucose utilisation and peripheral insulin sensitivity as well as by reducing intestinal glucose absorption and hepatic glucose generation.5 Lowering blood glucose with metformin in T2DM does not cause hypoglycaemia.6 Moreover, metformin was found to reduce weight in obese patients,7 improve lipid plasma levels,8 and prevent and delay progression of microvascular and macrovascular complications,2,5 which is of immense importance to reduce the risk of diabetes development and progression of overt T2DM in patients.
METHOD
A search strategy was developed to identify randomised controlled trials in both MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL). The terms “metformin”, “biguanides”, “fenformin”, “efficacy”, and “safety” were incorporated into an electronic search strategy that included the Dickersin filter for randomised controlled trials. The bibliographies of all identified randomised trials and review articles were reviewed to look for additional studies of interest. The author’s reviewed the citations retrieved from the electronic search to identify relevant articles for this review. The authors subsequently reviewed the potential trials to determine their eligibility. To qualify for inclusion, clinical trials were required to meet a series of predetermined criteria for study design, study population, interventions evaluated, and outcome measured. The following data were abstracted onto standardised case report forms: authors, year of publication, country of study, source of funding, study goal, means of randomisation and blinding, duration of treatment, treatment characteristics, sex, quantity of and reasons for study withdrawal, renal function and age characteristics of the treatment and control groups, outcomes, and adverse event data. A validated, three-item scale was used to evaluate the overall reporting quality of the trials selected for inclusion in the present review. This scale provided scoring for randomisation (0–2 points), double-blinding (0–2 points), and account for withdrawals (1 point). Scores ranged between 0 and 5, and scores of 3 or more indicated a study of high quality, and study selection was restricted to randomised controlled trials to ensure the inclusion of high-quality evidence only. In this review, the authors analysed the efficacy and safety of metformin in patients with CKD.
METFORMIN FORMULATIONS
Metformin reduces plasma glucose levels by acting at several different levels: it reduces hepatic glucose production in the liver by inhibiting gluconeogenesis and glycogenolysis, increases muscular insulin sensitivity and improves the uptake and utilisation of peripheral glucose, and it slows down the intestinal absorption of glucose.9 Until now, metformin was available as an immediate release (IR) formulation to be taken three times daily at a dosage of 500, 850, and 1000 mg, in tablet or in powdered form. The powder formulation was designed to overcome the challenge of considerable tablet size, which made them difficult to swallow, especially for elderly patients or people with dysphagia. The authors of this review have previously studied powder formulation and showed that the degree of patient satisfaction towards the antidiabetic treatment was increased and led to improved glycaemic control.10
Recently, extended release (XR) metformin has become available. Compared to conventional IR formulation, the XR offers some advantages. Firstly, the possibility to take the drug once a day, but with better gastrointestinal tolerability and equal effectiveness.11 The XR formulation has been designed to allow a more gradual release of the drug in the main absorption site, i.e., the upper gastrointestinal tract, thus improving its tolerability and patient compliance because of reduced administration frequency and a decrease in adverse events. However, apart from a review published on the comparison between metformin IR and metformin XR,11 few randomised clinical trials have been conducted to directly compare the two formulations. For example, Schwartz et al.12 conducted a study about a comparison between metformin XR treatment regimens versus metformin IR in a double-blind 24-week trial. Data showed that once- or twice-daily metformin XR was as safe and effective as twice-daily metformin IR and provided continued glycaemic control for up to 24 weeks of treatment. Similar results were reported by Fujioka et al.,13 who showed that patients with T2DM who had been receiving twice-daily metformin IR achieved comparable glycaemic control when therapy was switched to once-daily metformin XR, at the same or a greater total daily dose. Derosa et al.14 conducted a trial to compare metformin XR and metformin IR, recording a better effect of metformin XR compared to metformin IR in improving glycaemic control.
The same can be said about the lipid profile, with an improvement of total cholesterol and low-density lipoprotein cholesterol with metformin XR compared to IR. The positive effects of metformin on the lipid profile have been shown in rats.15 The authors also observed a visfatin improvement with metformin XR, not recorded with metformin IR. Visfatin is a protein expressed by adipocytes, and also by the liver, muscle, bone marrow, and lymphocytes.16 Visfatin exerts insulin-mimetic effects in cultured adipocytes, hepatocytes, and myotubes, and lowers plasma glucose in mice.17 Visfatin binds to the insulin receptor with similar affinity, but at a site distinct from insulin with insulin-sensitising effects. An improvement of visfatin improves insulin sensitivity.18
The better effects of metformin XR compared to metformin IR could be explained by better patient compliance and the minor incidence of adverse events.19 These data should not be surprising as one of the factors that affect glycaemic control is patient compliance to therapy. Patient compliance is related to the complexity of the treatment, total number of tablets taken daily, size of the tablets, difficulty in swallowing, side effects, and the cost of therapy.20
Timmins et al.21 obtained results for adverse events which were slightly different to those reported by Derosa et al.22 Timmins et al. found that adverse events with metformin XR were similar to those reported with metformin IR. However, they did not directly compare the two different formulations; moreover, they conducted the study in healthy subjects and not in patients with diabetes. Derosa et al. recorded that TNFα and high-sensitivity C-reactive protein were lower with metformin XR compared to baseline and to metformin IR, this could be attributed to the better improvement of glycaemic control obtained with metformin XR. It has already been shown that hyperglycaemia induces endothelial damage;22 postprandial glycaemia induces an acute, but repeated, systemic inflammation that could influence the development of cardiovascular disease in patients affected by disorders of glucose metabolism.23 Metformin XR better reduces glycaemic control with consequential minor endothelial damage and a reduction of inflammatory markers.
METFORMIN SAFETY
Metformin is a well-tolerated antidiabetic compound with additional metabolic protective effects, but there are some concerns for use of the drug in patients with T2DM with reduced renal function. There are no differences regarding renal safety among the different metformin formulations, even if many studies have been conducted on metformin IR and few on metformin XR, which were later available.
To understand the challenges for the use of metformin in patients with impaired renal function, it is beneficial to understand the pharmacokinetics of the compound. At a usual dosage of 500–1500 mg, metformin has an absolute oral bioavailability of 50–60%.24 The drug is not protein-bound and therefore has a wide volume distribution with maximum accumulation in the small-intestine wall. This biguanide is exclusively eliminated unchanged in the urine. Approximately 90% of absorbed metformin is eliminated via the renal route within the first 24 hours. In normal renal function, healthy people have an estimated glomerular filtration rate (eGFR) >90 mL/min/1.73 m2 with a plasma elimination half-life of metformin of approximately 5 hours, and there is minimal accumulation of the drug with multiple dosing.25 Compared with healthy individuals, patients with CKD show reduced metformin clearance which leads to an increase in metformin systemic exposure, increasing the risk of lactic acidosis (LA).26
Metformin decreases clearance of lactic acid by inhibiting the mitochondrial oxidation, thereby resulting in higher serum lactate concentrations.27-30 LA is defined as blood lactate concentrations >5 mmol/L and arterial pH <7.35.5 There are two forms of LA. Anaerobic LA (Type A LA), caused by lactate overproduction to regenerate ATP in the absence of oxygen, is usually seen in the presence of circulatory collapse, such as heart failure, sepsis, and shock. Aerobic LA (Type B LA), caused by underutilisation of lactate as a result of impaired removal by oxidation or gluconeogenesis, is associated with high anion gap, and is the type seen in liver disease, diabetes, cancer, and alcohol and metformin intoxication, or metformin-associated lactic acidosis (MALA). Combinations of Type A and B are possible. Lalau and Race31 have suggested that, since many cases of MALA are generally unrelated to metformin, the term MALA should be divided into metformin-unrelated LA and metformin-induced LA. The latter (Type B) is defined by raised metformin concentration and the risk of mortality is approximately 10%. Metformin-unrelated LA is primarily caused by Type A LA and bears a very high mortality of 50%.32-35
Even though toxic doses of metformin are a cause of LA, there are few data regarding the level predisposing to hyperlactataemia. Multiple studies suggest that elevated circulating lactate levels, often attributed to metformin, may actually not be caused by the drug. The therapeutic trough level for metformin is 0.7 (0.3–1.0) mg/L,36 while the pragmatic upper therapeutic limit is 5 mg/L.37 Intentional metformin overdose usually leads to hyperlactataemia, and often to LA. This can be fatal in cases with plasma metformin >50 mg/L.38 This has led to the sparsely science-based opinion that metformin is contraindicated for the treatment of patients with severe renal pathologies. There is no epidemiological evidence that metformin use increases the risk of LA. MALA is well described in case reports and case series throughout the literature. However, by most accounts, the risk of LA with therapeutic metformin use is considered minimal.
A Cochrane meta-analysis including 347 comparative controlled studies covering 70,490 patient-years, for those with T2DM, of metformin use revealed no cases of LA and no significant change in plasma lactate. Importantly, at the time this study was conducted, the use of metformin in patients with CKD was not permitted and only used in exceptional cases.39 No correlations were found between metformin and lactate levels. In this analysis, 53% of prospective studies allowed for inclusion of renal insufficiency, but patient-level serum creatinine concentrations were not always available for review. Based on statistical inference, the estimated upper limit of true incidence of LA was 4.3 cases per 100,000 patient-years. This investigation confirmed that LA is extremely rare.
A second meta-analysis was performed on all published studies in MEDLINE and Cochrane databases between 1950 and 2014 on 65 articles including pharmacokinetic/metabolic studies, large case series, retrospective studies, meta-analyses, and a clinical trial.40 The authors found that metformin plasma levels generally remained within the therapeutic range and lactate concentrations are not substantially increased when used in patients with moderate CKD (Stage 3). The overall incidence of MALA varied across studies from approximately 3 per 100,000 to 10 per 100,000 person-years and was generally indistinguishable from the background rate in the overall population with T2DM. The authors proposed a maximum metformin daily dose of 1,000 mg in patients with Stage 3b CKD. They also suggested that the risk of MALA is unlikely when the renal function remains stable and the patient is closely observed, even in patients with moderate CKD (eGFR: 30–59 mL/min). In this study, the authors showed that there is no risk.
Frid et al.41 observed the serum metformin levels of 137 patients with T2DM, of whom 20 had CKD (14 with Stage 3 and six with Stage 4), in a follow-up study for 2 months. There were few patients with metformin serum levels >20 μmol/L and median level was 10 μmol/L. The authors concluded that metformin may be safely used at an eGFR >30 mL/min and very high metformin levels are needed to cause LA. Lalau et al.42 conducted a study to define a safe, effective dose regimen for metformin in moderate and severe CKD (Stages 3a/3b and 4, respectively). After 4 months on these regimens, patients displayed stable metformin concentrations that never exceeded the generally accepted safe upper limit of 5.0 mg/L. Hyperlactataemia (>5 mmol/L) was absent (except in one patient with myocardial infarction), and glycated haemoglobin (HbA1c) levels did not change. There were no significant differences in pharmacokinetic parameters among the CKD stage groups. Further studies to assess the long-term safety of metformin in patients with T2DM with moderate renal impairment have not revealed increased risks in varying degrees of renal impairment.43-45
Taking into account the results of previous studies, Schernthaner and Schernthaner-Reiter46 calculated the hazard ratio (HR) of all-cause mortality for the use of metformin at different stages of CKD (Table 1).46 The authors recommended avoiding premature cessation of metformin therapy in patients with T2DM and CKD to counter poor glucose control and further increase in the already high risk of cardiovascular disease. Based on their meta-analysis and available data on efficacy and safety, they recommended the use of metformin in patients with CKD including Stage 3b, up to 1,000 mg/day, but not in Stage 4.
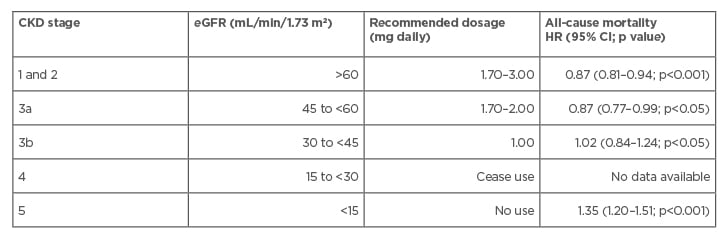
Table 1: Use of metformin during different stages of chronic kidney disease.
CI: confidence interval; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HR: hazard ratio.
METFORMIN, TYPE 2 DIABETES MELLITUS, AND ADVANCED CHRONIC KIDNEY DISEASE
Globally, drug regulatory agencies have issued specific cautions and restrictions related to the use of metformin in patients with T2DM and advanced CKD. The concrete metabolic and cardiovascular benefits associated with metformin therapy derived from clinical and scientific evidence have encouraged some authors to extend the therapy options to patients with CKD Stage V, both in dialysis and conservative treatment.
Hung et al.47 conducted a retrospective observational cohort study on patients with T2DM and CKD Stage 5 using Taiwan’s National Health Insurance Research Database (NHIRD) between 2000 and 2009. Approximately 8% of patients (12,350) were using metformin despite contraindication and were matched at a ratio of 1:3 with nonusers by propensity score and followed-up for 2.1 years. After multivariate adjustment, metformin use was associated with a higher, but nonsignificant, risk of metabolic acidosis of 1.6 versus 1.3 events per 100 patient-years (adjusted HR: 1.3, p=0.19), and no dose correlation was observed (HR: 1.8 in ≤500 mg/day; 1.4 in 500–1,000 mg/day; and 1.5 in ≥1,000 mg/day).47 In patients using metformin, ESRD was significantly lower (HR: 0.76, p<0.0001) in comparison to the control group and metformin was associated with an increased mortality (HR: 1.35, p<0.001) in a dose-dependent manner (HR: 1.14 in ≤500 mg/day; 1.30 in 500–1,000 mg/day; 1.57 in ≥1,000 mg/day). After this study, the Taiwan National Health Insurance (NHI) announced that metformin use was contraindicated in females and males with serum creatinine concentrations of >1.5 and >1.4 mg/dL, respectively.
In a pilot experience, 35 patients on automated peritoneal dialysis with T2DM were treated with metformin, despite their very low eGFR.48 After 11 months of treatment with metformin at doses 0.5–1.0 g/day, a reduction of 7.4% to 6.4% HbA1c, 1.5 kg/m2 in BMI, and -30% insulin requirements was observed. Metformin concentrations were elevated in 81% of samples and markedly elevated (>5 mg/L) in 4% of samples, no change in anion gap or pH was seen, and only 0.76 % of blood samples had a plasma lactate >2 mmol/L. There was no correlation between metformin concentration and lactate and no cases of LA. The authors suggested that peritoneal dialysis, by causing rapid removal of lactate and restitution of acid base balance, may protect against LA itself.
COMPLICATIONS OF METFORMIN IN DIABETIC NEPHROPATHY
Another important issue regarding metformin use concerns kidney transplant patients. Nondiabetic kidney transplant recipients are at risk for developing new onset diabetes after transplant, a common complication associated with kidney transplant that can affect allograft and patient survival.49 To prevent complications associated with diabetes, proper glycaemic control is imperative; however, the extent of metformin use among kidney transplant recipients is currently uncertain. In 2008, Kurian et al.50 demonstrated that metformin was safe in 24 kidney transplant recipients for a mean duration of 16.4 months up to a maximum of 55 months.50 Although the study found no cases of LA, eGFR decreased in all patients. Patients with pre-existing diabetes experienced significant changes in eGFR. More recently, an observational study showed that 9.8% of kidney transplant recipients who filed at least one prescription for an antiglycaemic agent also had at least one claim for metformin or a metformin-containing agent.51 Metformin was associated with lower adjusted HR for both living donors and deceased donor allograft survival at 3 years post-transplant, and with lower mortality.
The many risks for LA in patients with renal impairment could be partially circumscribed to specific predisposing risk factors. Renal function is dynamic, and renal dysfunction in T2DM is typically progressive. Thus, the renal thresholds for the acceptability of metformin therapy should ideally account for the stage in the CKD progression. The renal thresholds for prescription of metformin therapy should consider the stage and progression of CKD. The assessment of renal function in clinical practice occurs periodically, and the degree of renal dysfunction may change appreciably between these assessments. Therefore, it is essential to know how quickly eGFR declines in the typical spectrum of nephropathy among patients with T2DM, particularly when considering metformin therapy.
The most common side-effects observed in association with metformin use in patients with T2DM with mild to moderate renal impairment are gastrointestinal events including diarrhoea, nausea, vomiting, abdominal pain, and decreased appetite, among others. Few studies have, however, systematically evaluated the effect of rate of progression of renal dysfunction and the risk of LA in the diabetic population.
In a matched case control study conducted by Grenoble Hospital University Center, La Tronche, France, to evaluate the strength between the association between LA and well-recognised risk factors,52 metformin was not associated with a higher risk of LA in patients with T2DM. Metformin was significantly associated with a higher LA probability in cases of acute kidney injury (odds ratio [OR]: 1.79, p<0.02) but not in patients without acute kidney injury. Intercurrent diseases such as acute decompensated heart failure, acute respiratory failure, and sepsis, were significantly associated with LA (OR: 3.55, p<0.001; 9.58, p<0.001; and 8.28, p<0.001, respectively), while other chronic medical conditions had a minor impact on LA incidence, except hepatocellular dysfunction.
Special attention should be given to contrast-induced nephropathy, a common complication after administration of iodinated contrast media. Metformin, by itself, is not a risk factor for contrast-induced nephropathy,53 but the risk of acute renal function deterioration increases the risk of acute kidney injury, which is the main risk factor for metformin accumulation.54 In a cohort study,55 which included patients with T2DM with moderate CKD (eGFR <60 mL/min) under metformin treatment, no significant changes in renal function were observed after endovenous administration of iodinated contrast. However, the authors’ optimistic conclusions were limited by the low sample size and the retrospective study design. Lepelley et al.52 found a higher risk for LA with the use of contrast media (OR: 8.58, p<0.001) compared to metformin (OR: 1.79, p=0.02).
Based on the European Society of Urogenital Radiology (ESUR),56 patients receiving endovenous iodinated contrast should stop taking metformin 48 hours before contrast administration if their eGFR falls <45 mL/min. Renal function should be revalued 48 hours after contrast administration and metformin should only be restarted if it has not deteriorated further. The Canadian Association of Radiologists (CAR) uses a threshold of <60 mL/min.53
CURRENT GUIDELINES AND FUTURE IMPLICATIONS
These studies highlight the lack of randomised clinical trials to test the specific hypothesis that metformin is safe in patients with mild to moderate CKD. Randomised trials would help to better inform evidence-based guidelines. Nevertheless, given the rarity of LA in the setting of metformin therapy, a study would need to examine hundreds of thousands of patients for many years to demonstrate noninferiority compared with other hypoglycaemic agents, which might not be feasible. National patient registries might be a reasonable alternative; however, for regulatory bodies at this time, the best available evidence is limited to meta-analyses, retrospective studies, and smaller mechanistic investigations reported herein.
Contraindications to the use of metformin are based on the cut-off points of serum creatinine values, discouraging its use at or above the 1.4 and 1.5 mg/dL levels in females and males, respectively. In any case, the current recommendations for metformin are not clear and univocal for advanced CKD. The latest Kidney Disease Outcomes Quality Initiative guidelines recently updated by the National Kidney Foundation (NKF KDOQI) are perfectly in line with this criterion.2 Despite this, some practice guidelines present substantial differences for the use of metformin in renal patients. In the ADA guidelines, for example, renal thresholds are actually not discussed.4 In the statement position of the ADA and EASD,57 the members reports are that metformin appears to be safe unless the noninferior eGFR fall to 30 mL/min for 1.73 m2.
Other non-American guidelines considered the use of eGFR to determine the safety of metformin. The National Institute for Health and Care Excellence (NICE) recommends using metformin with caution in patients58 for whom serum creatinine >130 μmol/L (1.47 mg/dL) or eGFR <45 mL/min. Doses should be lower and prescribed with increased frequency of monitoring. In patients already taking metformin, the drug should be discontinued if the serum creatinine >150 μmol/L (1.70 mg/dL) or GFR <30mL/min.
Other associations such as the Canadian Diabetes Association (CDA)58 and Australian Diabetes Society (ADS) practice guidelines are now based solely on eGFR, recommending caution with eGFR of 60 mL/min per 1.73 m2 and contraindicating its use with eGFR of 30 mL/min per 1.73 m2.59 The European Renal Association/European Dialysis and Transplant Association (ERA-EDTA) have recently published the clinical practice guideline in the management of patients with diabetes and CKD Stage 3b or higher (eGFR <45 mL/min).60 Metformin is recommended as a first-line agent in a dose adapted to the renal function, when lifestyle measures alone are insufficient to lower HbA1c to the desired range. The committee has based their recommendation on the most positive benefit amongst all treatment classes. A maximum daily dose of 850–1,500 mg/day for CKD Stage 3b is suggested. In CKD Stage 4, 500 mg/day should not be exceeded.
The Kidney Disease Improving Global Outcomes (KDIGO) guideline proposed that the dose of metformin should be reduced to a maximum of 1,000 mg/day when eGFR reaches 45 mL/min, and should generally be discontinued when eGFR reaches 30 mL/min.61 The use of metformin may be appropriate in patients with even more advanced CKD (eGFR 15–29 mL/min) if the kidney disease is stable and if alternative treatments to manage glycaemia are unavailable or produce significant side effects.
CONCLUSION
Although different formulations of metformin have been evaluated in recent years, generally, metformin is a bulwark in the treatment of diabetes, and it is also currently recommended for patients with nephropathy by monitoring renal function. There is clear recognition that renal failure may be a risk factor for adverse events with metformin use, even if there is a significant divergence in opinion around the world regarding the optimal definition of safety. Provided that the dose is adjusted for renal function, metformin treatment appears to be safe and pharmacologically efficacious in moderate-to-severe CKD.








