Abstract
Diabetic macular oedema (DMO) is the leading cause of vision loss in working aged individuals. Macular laser photocoagulation was the primary DMO treatment for several decades, but has recently been replaced by intravitreal injections of corticosteroids and drugs that inhibit the actions of vascular endothelial growth factor (VEGF). In Phase III trials, anti-VEGF drugs improve best corrected visual acuity by a mean of +12 letters, but up to 40% of patients have sub-optimal responses to therapy. The new anti-VEGF drugs abicipar and brolucizumab may possess extended durations of action in Phase III neovascular age-related macular degeneration trials, and DMO trials are being planned. Angiopoietin-2 inhibitors, both as co-formulations with anti-VEGF drugs and as bispecific antibodies, are in Phase II trials for DMO. Drugs that stimulate the Tie2 receptor are administered via subcutaneous injections. Intravenously administered antibodies that decrease diabetes-mediated inflammation, such as tocilizumab and teprotumumab, are entering early phase studies. Other drugs with topical (mecamylamine) and oral (minocycline) delivery routes are being developed. Several of these drugs may become available to patients within the next 5–10 years.
INTRODUCTION
Diabetic retinopathy (DR) is the leading cause of blindness in working-aged individuals in industrialised nations1 and 75% of these cases are the result of diabetic macular oedema (DMO).2 Chronic hyperglycaemia dysregulates several biochemical pathways (hexosamine, aldose reductase, advanced glycation end-products, and protein kinase C) and leads to the accumulation of abnormal by-products that interfere with electron transfer through the cytochrome chain within the mitochondria.3 The resultant superoxides induce chronic inflammation by upregulating several chemokines and cytokines, including interleukin (IL)-1β, IL-6, interferon-inducible protein 10, intercellular adhesion protein-1, monocyte chemotactic protein-1, placental growth factor, and vascular endothelial growth factor (VEGF).4 Chronic inflammation activates retinal glial cells; damages capillary endothelial cells5 and pericytes,6,7 thereby producing neurodegeneration;8 and, together with advanced glycation end-products, thickens vascular basement membranes.9 Blood-retinal barrier breakdown enables albumin and water to pass into the retinal interstitium, resulting in the formation of DMO.10
Laser photocoagulation of microaneurysms and leaking retinal capillary beds had been the standard treatment of DMO for three decades11 but drugs that inhibit VEGF have recently become first-line therapy for centre-involving DMO.12,13 They improve mean best corrected visual acuity (BCVA) by ≤13 letters and decrease macular thickness;12-14 however, 20–40% of patients respond poorly to repeated intravitreal injections.15
The most potent DMO treatments require repeated intravitreal injections, but compliance with these regimens can be challenging. Drugs that can be administered topically, subconjunctivally, subcutaneously, and orally may be easier to administer, but questions regarding ocular penetration, efficacy, and systemic safety must be addressed. New medications that work synergistically with existing drugs, or serve as salvage therapy when eyes fail to respond sufficiently to first-line therapies, are needed to improve overall efficacy.
DRUGS UNDER DEVELOPMENT
VEGF plays a central role in blood-retinal-barrier breakdown in most eyes with DMO. Modulation of other molecular targets, however, may also produce important clinical responses. Drugs that are being evaluated in both preclinical studies and early phase clinical trials are detailed in the following sections.
Vascular Endothelial Growth Factor Inhibitors (Table 1)
Abicipar pegol
Abicipar pegol (Allergan, Irvine, California, USA), a designed ankyrin repeating protein, is currently being developed for the treatment of oncologic, inflammatory, and chorioretinal vascular conditions.16 Abicipar binds all isoforms of VEGF-A with a high affinity (KD=2 pM for VEGF165) and, because of its polyethylene glycol moiety, possesses a long intravitreal half-life in rabbits (6 days). A Phase I/II, multicentre, open-label, dose-escalation trial determined that singular 1 mg injections produced excellent reductions in DMO-related macular thickness and improvements in mean BCVA (+10 letters) at 12 weeks.17 Pharmacokinetic analyses of anterior chamber drug concentrations suggested that abicipar has a long intraocular half-life (13.4 days) in humans. A recently completed Phase II DMO trial with once every 8 weeks and once every 12 weeks dosing met its primary (BCVA improvements of +7.1 and +7.2 letters) and secondary (central retinal thickness improvements of -158.8 µm and -162.0 µm) endpoints.18 Data from the Phase II trial supports progression to Phase III.19
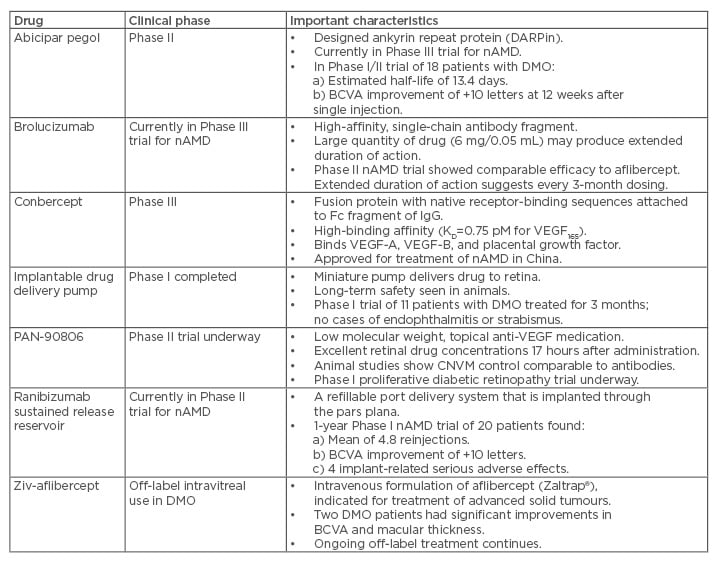
Table 1: Vascular endothelial growth factor inhibitory drugs being evaluated for the treatment of diabetic macular oedema.
BCVA: best corrected visual acuity; CNVM: choroidal neovascular membrane; DMO: diabetic macular oedema; Ig: immunoglobulin; nAMD: neovascular age-related macular degeneration; VEGF: vascular endothelial growth factor.
Brolucizumab
Brolucizumab (RTH258, Alcon, Fort Worth, Texas, USA) is a single-chain, VEGF-binding, antibody fragment being developed for the treatment of neovascular age-related macular degeneration (nAMD). Its small molecular weight allows large drug quantities to be injected; as such, developers hope that brolucizumab will have a longer duration of action than currently available anti-VEGF drugs.
A Phase II trial compared 6 mg brolucizumab to 2 mg aflibercept in patients with nAMD.20 At the study’s 12-week primary endpoint, brolucizumab produced BCVA gains that were non-inferior to aflibercept and with a greater reduction in macular fluid. Patients treated at 12-week intervals improved, suggesting that the drug possesses a long duration of action.20
Conbercept
Conbercept (KH902, Chengdu Kanghong Biotech, Sichuan, China) is a 143 kDa, recombinant, fusion protein that contains the second immunoglobulin (Ig) binding domain from VEGF receptor 1 (VEGFR1), the third and fourth binding domains from VEGFR2, and the fragment crystallisable region of human IgG.21 The fourth Ig domain of VEGFR2 enhances the association rate of VEGF to the receptor and is essential for receptor dimerisation.conbercept possesses a high affinity for VEGF. Conbercept mimics aflibercept by acting as a soluble receptor that binds all isoforms of VEGF-A, VEGF-B, and placental growth factor. Conbercept has already been approved for the treatment of nAMD in China and a Phase III trial evaluating its efficacy for the treatment of DMO is currently enrolling patients.22
Implantable drug delivery pump
The Posterior MicroPump Drug Delivery System (PMP, Replenish Inc., Pasadena, California, USA) uses a microelectromechanical system technology (the same technology used in insulin pumps) to deliver drugs into the eye. The PMP can reliably deliver 100 programmed doses of an anti-VEGF drug (equivalent to >8 years of therapy) into animal eyes and long-term safety has been demonstrated.23 The PMP was well tolerated for 3 months by 11 patients with DMO, with no cases of endophthalmitis or strabismus.24
PAN-90806
A low molecular weight VEGF receptor blocker (PAN-90806, PanOptica, London, UK) produces excellent drug concentrations in the central retina and choroid up to 17 hours after topical administration, but with minimal systemic exposure. Decreased leakage and bleeding from choroidal neovascular membranes, comparable to that achieved with intravitreal anti-VEGF antibodies, have been seen in animal studies. Patients in each of the four monotherapy arms in a Phase I/II nAMD trial experienced improved visual acuity.25 A Phase I trial for proliferative DR has been completed but the results have not been reported.26
Ranibizumab sustained release reservoir
A refillable ranibizumab port delivery system continuously releases ranibizumab into the vitreous to reduce the need for repeated intravitreal injections. The preloaded implant is surgically implanted through a 3.2 mm pars plana incision and the port, which is covered with conjunctiva, can be accessed and refilled in the office.
In a Phase I nAMD trial,27 the reservoir was implanted and eyes were injected with 0.5 mg ranibizumab: 0.25 mg into the vitreous and 0.25 mg into the reservoir. Additional injections were given according to optical coherence tomography-based assessment of disease activity. Four of the 20 patients had significant or serious adverse events (endophthalmitis [n=1], vitreous haemorrhage [n=2], and traumatic cataract [n=1]), but 3 of these 4 had improved BCVA by the study’s 12-month endpoint. The average BCVA gains for the cohort were +10 letters, with 10 eyes (50%) gaining at least 3 lines and 2 (10%) losing at least 3 lines. The mean number of refills was 4.8 per patient. The ongoing Phase II trial is using 0.75 mg injections in an attempt to extend the treatment intervals to 4 months.28
Ziv-aflibercept
Ziv-aflibercept (Zaltrap®, Regeneron, Tarrytown, New York, USA) is the intravenous formulation of Eylea® (a VEGF-A, VEGF-B, and placental growth factor blocker) that is used to treat advanced colorectal carcinoma. Small cohorts of nAMD patients that received single injections of ziv-aflibercept experienced improvements in thickness and BCVA at 1 month without evidence of toxicity.29 Two patients with DMO experienced improved BCVA (20/800 to 20/100, and 20/800 to 20/200, for each patient, respectively) and macular thickness (central subfield thickness [CST]: -65 µm and -352 μm, respectively) 1 week after intravitreal injections.30 Investigational ziv-aflibercept treatment of patients with nAMD, DMO, and retinal vein occlusions continues.
OTHER TREATMENTS (TABLE 2)
Adenosine Kinase Inhibitor
The selective adenosine kinase inhibitor, ABT-702, was injected twice-weekly into streptozotocin-induced diabetic mice31 and retinal inflammation was evaluated using a Western blot, real-time polymerase chain reaction, and immuno-staining analyses. The role of A2A adenosine receptor signalling was analysed in amadori-glycated-albumin-treated microglial cells. At 16 weeks, when diabetic mice usually exhibit significant signs of retinal inflammation, including upregulation of oxidative/nitrosative stress, A2AAR, ENT1, Iba1, tumour necrosis factor (TNF)-α, ICAM-1, retinal cell death, and downregulation of adenosine kinase, the ABT-702 treated group showed decreased signs of inflammation compared to control animals receiving the vehicle.
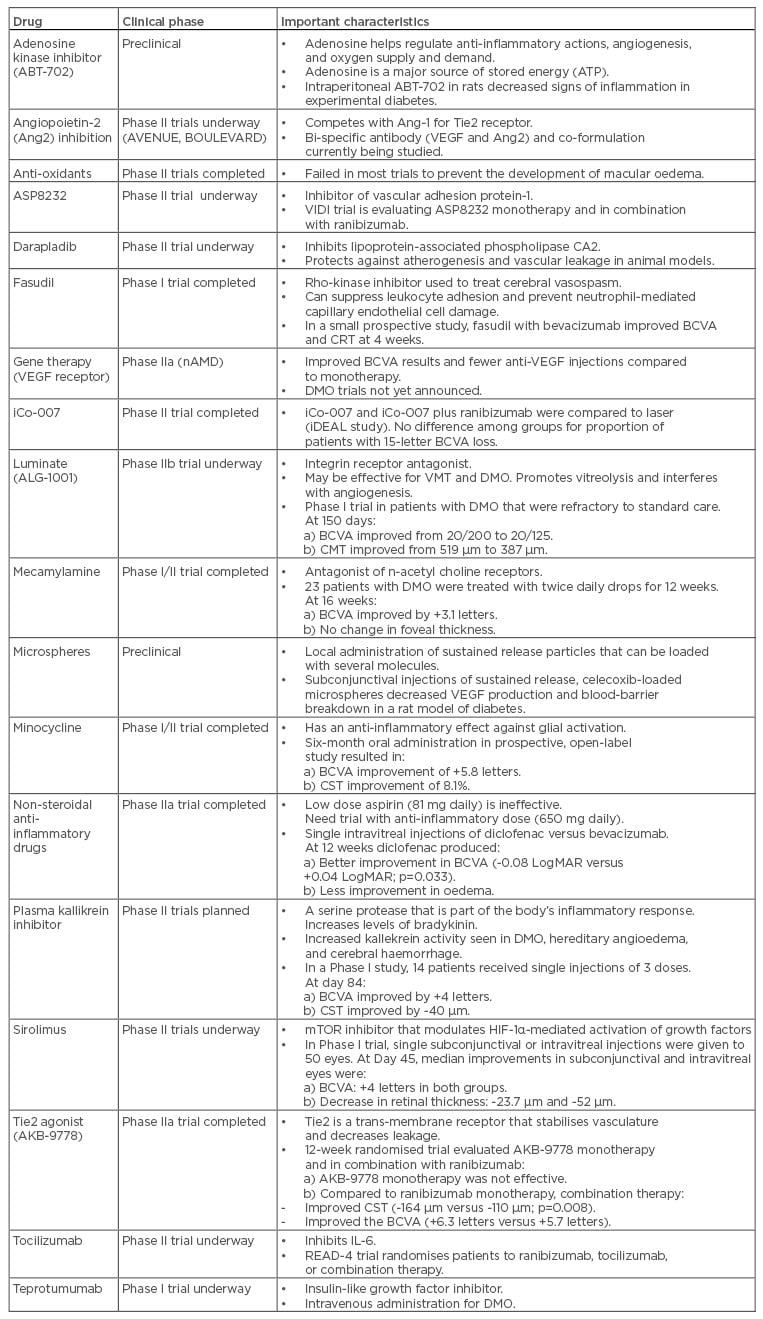
Table 2: Drugs not in the previously identified categories being evaluated for the treatment of diabetic macular oedema.
BCVA: best corrected visual acuity; CMT: central macular thickness; CRT: central retinal thickness; CST: central subfield thickness; DMO: diabetic macular oedema; IL: interleukin; logMAR: Logarithm of the Minimum Angle of Resolution; mTOR: mechanistic target of rapamycin; VEGF: vascular endothelial growth factor; VMT: vitreomacular traction.
Angiopoietin-2 Inhibition
Angiopoietin-2 (Ang2) promotes angiogenesis and vascular leakage in the presence of VEGF and proinflammatory cytokines, but facilitates vascular regression in the absence of VEGF.32 Ang2 sensitises endothelial cells to TNF-α induced expression of ICAM-1, the critical player in the pathogenesis of inflammation-induced retinopathy.33 Intravitreal injection of Ang2 into non-diabetic rats increases retinal vascular permeability.34 Pharmacologic blockade of Ang2 might also prevent pericyte dropout in DR.35
Elevated vitreous concentrations of Ang2 have been found in patients with DMO.36 A bispecific-anti-VEGF and anti-Ang2 antibody is currently in Phase II testing for patients with DMO (BOULEVARD trial, Hoffman-La Roche, Basel, Switzerland)37 and an anti-Ang2 antibody is being injected with aflibercept (AVENUE Trial, Regeneron).38
Anti-Oxidants
Evidence from animal studies both supports and refutes the use of antioxidants to prevent experimental diabetic retinopathy,39,40 but this use of antioxidants has not been supported by clinical trials in humans.41
ASP8232
ASP8232 belongs to a novel class of orally administered vascular adhesion protein-1 inhibitors that is being evaluated in a Phase II, multicentre, randomised controlled trial (the VIDI study) for the treatment of DMO. The safety and efficacy of ASP8232 plus sham are being compared to ASP8232 plus ranibizumab and placebo plus ranibizumab. Enrolment (84 patients) was completed in 2016, but results have yet to be posted.42
Darapladib
Darapladib, a lipoprotein-associated phospholipase CA2 (Lp-PLA2) inhibitor, protects against atherogenesis and vascular leakage in diabetic and hypercholesterolaemic animal models. It suppresses blood-retina barrier (BRB) breakdown in streptozotocin-diabetic Brown Norway rats, comparable to that achieved with intravitreal anti-VEGF therapy.43 In a Phase IIa study, patients receiving darapladib experienced improvements in BCVA (+4.1 letters) and CST (-57 µm) compared to the placebo group.44
Fasudil
Fasudil (Asahi Kasei Pharma Corporation, Tokyo, Japan), a rho-kinase inhibitor used to treat cerebral vasospasm after aneurysm rupture and stroke, primary pulmonary hypertension, and memory deficits in patients with Alzheimer’s disease suppresses leukocyte adhesion, prevents neutrophil-induced retinal capillary endothelial cell damage,45 and may directly protect vascular endothelial cells by reversing endothelial cell nitric oxide synthase activity.
In a small, prospective study, patients with DMO received single intravitreal injections of bevacizumab combined with fasudil (0.025 mg). Compared to baseline, patients had significant improvements in mean BCVA (0.84 Logarithm of the Minimum Angle of Resolution [logMAR] to 0.49 logMAR; p=0.003) and mean central retinal thickness (448 µm to 347 µm; p=0.001) at 4 weeks.46
Gene Therapy
Management of DR with gene therapy has been proposed for several years; it is thought that effective therapies may improve efficacy, decrease the need for frequent injections and clinic visits, improve patient compliance, decrease side effects, and allow intervention to be performed earlier in the disease process (i.e. during retinal neurodegeneration). Unfortunately, advances in research have been slow and therapies are not yet available. Gene therapy for inherited retinal disorders, such as Leber’s congenital amaurosis, Stargardt’s disease, X-linked retinoschisis, and choroideremia, is underway. The most important biochemical target in patients with DMO is VEGF, and single injections of a gene coding for a soluble VEGF receptor have produced encouraging results in patients with nAMD,47 but DMO trials have not yet been performed.
Other chemokines and cytokines (endostatin, pigment epithelium derived factor, hypoxia inducible factor-1α, angiostatin) have been targeted in animal models, but few studies have been performed in humans. Significant efforts have been made to identify other genetic factors that predispose to DR, but few consistent findings have emerged.48
iCo-007
The anti-sense oligonucleotide iCo-007 inhibits c-Raf expression and blocks mitogen-activated protein kinase signalling. iCo-007 has a favourable ocular pharmacokinetic profile with an intraocular half-life of 6–8 weeks in rabbits and monkeys after intravitreal injection.49
In a Phase I, dose-escalation study (doses ranging between 110 µg and 1,000 µg), 15 patients with diffuse DMO received single, intravitreal injections of iC0-007. At the 24-week secondary endpoint, mean reduction of excess retinal thickness was 40%, and 69% of patients experienced improved BCVA.50 A multicentre, Phase II trial evaluated iCo-007 monotherapy and combination therapy with ranibizumab or laser for centre-involving DMO (the iDEAL Study). At 8 months, BCVA improved by 15 letters in 64% (700 µg monotherapy arm), 33% (350 µg monotherapy arm), 33% (350 µg plus laser arm), and 41% (350 µg plus ranibizumab arm) of patients, whereas at 4 months the results were 29%, 9%, 9%, and 14%, respectively.51
Luminate (ALG-1001)
Luminate (ALG-1001, Allegro Ophthalmics, San Juan Capistrano, California, USA) is a first-in-class therapy that targets integrin receptors involved in cell signalling and regulation, and in the formation of new blood vessels. ALG-1001 exhibits prolonged binding to all integrin receptors involved with retinal angiogenesis.52 Luminate may be useful to treat both vitreomacular traction (by promoting vitreolysis) and macular vascular diseases (by interfering with angiogenesis).
A Phase I study evaluated the safety and efficacy of luminate in 15 subjects with advanced DMO. Patients received three 2.5 mg intravitreal luminate injections at monthly intervals, with 3 months follow-up after the last injection. No subjects lost BCVA or experienced an increase in CST, and no significant adverse events were seen during follow-up. Mean BCVA improved from 20/200 at baseline to 20/125 at 60 days (last treatment) and remained stable throughout 150 days. The mean central macular thickness decreased from 519 μm to 387 μm at 150 days.53 Luminate is being evaluated in a Phase IIb DMO clinical trial against bevacizumab and focal laser. The enrolment goal (150 patients) was met in late 2015.54
Mecamylamine
In a multicentre, Phase I/II DMO trial, the safety and bioactivity of topical 1% mecamylamine, an antagonist of nicotinic acetylcholine receptors, was tested in 23 patients.55 Mecamylamine 1% drops were well tolerated when administered twice-daily and there were no drug-related safety problems. Mean improvements in BCVA at 1, 4, 8, 12, and 16 weeks were +2.8, +1.9, +2.4, +0.8, and +3.1 letters, respectively. There was little change in mean excess foveal thickness, but there was substantial heterogeneity in response since 8 patients had improved BCVA, foveal thickness, or both, 9 patients experienced no significant changes, and 4 patients worsened. The study suggested that the effects of topical mecamylamine are heterogeneous in patients with DMO.
Microspheres
Administration of biodegradable microspheres may be an attractive alternative to frequently repeated injections since they can deliver drugs in a controlled way. Most treatable retinal diseases are the result of several biochemical abnormalities, and as such microspheres represent a promising treatment platform that can be filled with several active substances. Microsphere carriers have been loaded with budesonide and celecoxib to treat experimental DR in rats.56 A posterior subconjunctival injection (0.05 mL) of the celecoxib-microsphere suspension inhibited diabetes-induced VEGF elevations and BRB leakage in rat eyes.57
Minocycline
Neuroretinal inflammation usually precedes the microvascular findings in DR and activates microglia.58 Tetracycline reduces inflammation-mediated connective tissue breakdown, protein glycation, and excessive collagen synthesis, and limits microglial-mediated cell death, retinal cell apoptosis, and capillary damage by inhibiting caspase.59 Minocycline, a commonly used second-generation tetracycline, has anti-inflammatory properties that are independent of its antibacterial property.59 Oral minocycline (100 mg twice-daily for 6 months) was studied in a single-centre, prospective, open-label, Phase I/II clinical trial of 5 participants with fovea-involving DMO.60 Mean BCVA improved continuously from baseline through 1, 2, 4, and 6 months by +1.0, +4.0, +4.0, and +5.8 letters, respectively, while CST decreased by 2.9%, 5.7%, 13.9, and 8.1%, respectively, at the same time points. At 6 months, the mean area of late leakage on fluorescein angiography decreased by 34.4% in study eyes. Two trials with oral doxycycline, however, produced conflicting results.61,62
Non-Steroidal Anti-Inflammatory Drugs
In clinical studies, low-dose aspirin (81 mg) has shown little or no benefit in preventing DR. Further work is still needed, however, to determine if high-dose aspirin (650 mg), which has a greater anti-inflammatory effect, can prevent the development of DR. In a randomised trial, 57 eyes with treatment naïve DMO received single intravitreal injections of either diclofenac (500 μg/0.1 ml) or bevacizumab.63 At the 12-week endpoint, eyes receiving diclofenac had better mean improvements in BCVA compared to bevacizumab (Δ -0.08 LogMAR versus Δ +0.04 LogMAR; p=0.033), but bevacizumab improved macular oedema slightly better.
Plasma Kallikrein Inhibitor
Plasma kallikrein activity is upregulated in many diseases, including DMO, hereditary angioedema, and cerebral haemorrhage; several components of the kallikrein-kinin system, including plasma kallikrein, factor XII, and kininogen, have been found in the vitreous of patients with advanced DR.64 Rodent studies have shown that activated intravitreal plasma kallikrein increases retinal vascular permeability, whereas kallikrein inhibition reduces diabetes and hypertension-induced retinal vascular leakage.65
A low molecular weight, plasma kallikrein inhibitor (KVD001) to treat DMO and hereditary angioedema is currently being developed. A 5-site, Phase I DMO study treated three cohorts (a total of 14 patients) that had previously received anti-VEGF injections. Single injections of 1, 3, and 10 µg KVD001 improved mean BCVA and CST by +4 letters and -40 µm, respectively, at day 84.66 Two Phase II trials are being planned, one combining a plasma kallikrein inhibitor with an anti-VEGF drug, and the other evaluating plasma kallikrein inhibitor monotherapy in eyes with anti-VEGF resistant DMO.67
Sirolimus
The mechanistic target of rapamycin (mTOR) inhibitors may delay or prevent breakdown of the BRB and progression of retinal microangiopathies by modulating HIF-1α-mediated downstream activation of growth factors.68 As DR progresses and proliferative lesions develop, PI3K/Akt/mTOR pathway inhibition may promote neovascular regression by downregulating pro-survival growth factors, modulating the inflammatory cascade, preventing angiogenesis, and promoting apoptosis of newly formed vessels.69
A randomised, open-label, dose-escalating, Phase I study evaluated the safety and tolerability of sirolimus (Perceiva, Macusight, Union City, California, USA) for the treatment of DMO.70 Single subconjunctival (220, 440, 880, 1320, or 1760 µg) or intravitreal (44, 110, 176, 264, or 352 µg) injections were given to 50 eyes of 50 patients.
No dose-limiting toxicities were observed and ocular adverse events were mild and transient. For the subconjunctival group, median increases in BCVA at Days 7, 14, 45, and 90 were +5, +3, +4, and +4 letters, respectively. Median decrease in retinal thickness was -23.7 µm at Day 45. For the intravitreal group, median increase in BCVA was +2 letters, which was maintained throughout the 90 days (+4.0 letters); the median decrease in retinal thickness was -52.0 µm at Day 45. These findings support advancing the present sirolimus formulation into Phase II studies.
Tie2 Agonist (AKB-9778)
Tyrosine kinase with Ig-like and EGF-like domains 2 (Tie2) is a transmembrane receptor that regulates vascular permeability.71 Activation of Tie2 stabilises vasculature and decreases leakage. Angiopoietin-1 stimulates Tie2 phosphorylation,72 whereas angiopoietin-2 only partially stimulates Tie2 phosphorylation and, therefore, competes with angiopoietin-1 to suppress Tie2 phosphorylation.73
A randomised, placebo and sham injection-controlled, double-masked, Phase IIa DMO trial assessed the effect of the Tie2 agonist AKB-9778 (administered subcutaneously twice-daily) alone or in combination with ranibizumab in 144 patients. At 12 weeks, the mean change in CST was significantly greater in the combination group compared with the ranibizumab monotherapy group (-164 μm versus -110 μm; p=0.008) but was only -6 μm in the AKB-9778 monotherapy group. The mean change in BCVA was +6.3 letters in the combination group, +5.7 in the ranibizumab monotherapy group, and +1.5 in the AKB-9778 monotherapy group. Improvements in DR severity scores were similar across groups and the percentage of qualified fellow eyes with a ≥2-step improvement was 11.4% in all AKB-9778-treated patients compared with 4.2% in the ranibizumab monotherapy group. The authors concluded that activation of Tie2 by subcutaneous injections of AKB-9778 combined with VEGF suppression reduces DMO greater than with anti-VEGF monotherapy.74
Tocilizumab
The READ-4 study will compare ranibizumab and tocilizumab, an IL-6 inhibitor, in the treatment of DMO. The study will randomise patients to receive ranibizumab, tocilizumab, or a combination with the primary endpoint analysis at 6 months. Enrolment for the study is expected to begin in the final quarter of 2017.75
Teprotumumab
Currently the intravenously administrated insulin-like growth factor-1 inhibitor teprotumumab (RV001) is being evaluated in an open-label Phase I study at three centres.76
CONCLUSION
A robust pipeline features numerous DMO-treating drugs in various stages of development. Drugs are being evaluated both as monotherapy and in combination with anti-VEGF therapy. Regardless of the successes of these new drugs, however, anti-VEGF treatment will likely remain an important component of DMO therapy for many years.








