Abstract
Introduction: Excess body weight and low physical activity levels may be detrimental to cancer survivorship and to the development of diabetes and cardiovascular disease (CVD). This study aimed to test the feasibility and acceptability of an adapted Diabetes Prevention Programme (DPP) for cancer survivors who have risk factors for Type 2 diabetes mellitus and CVD.
Methods: Overweight (BMI >25 kg/m²) adults aged 50–79 who were diagnosed with nonmetastatic breast or colon cancer within the prior 5 years were recruited through a research registry and oncology clinics. Eligible individuals enrolled in a 13-week group lifestyle programme with goals of 5–7% weight loss and 150 minutes of moderate-intensity physical activity. Programme attendance, adherence to recommended behaviours, weight, and physical activity information were collected.
Results: A total of 44 individuals were screened for eligibility; 23 were eligible and 17 enrolled in the programme. Participants attended a median of 10 out of 13 lifestyle sessions and were able to meet dietary and activity goals 72.7% and 56.3% of the time, respectively. At the end of the programme, median weight loss was 4.5% and median activity was 297 minutes/week (median change +164 minutes/week).
Conclusion: The modified DPP intervention was feasible to deliver to this group of cancer survivors who had risk factors for diabetes or CVD. Incorporating successful prevention programmes such as the DPP into cancer survivorship care has the potential to improve health behaviours and chronic disease risk factors in the cancer survivor population.
INTRODUCTION
There are currently 14 million cancer survivors in the USA, a number that is expected to increase as the population ages.¹ Competing health risks associated with ageing, such as Type 2 diabetes mellitus and cardiovascular disease (CVD), require special consideration because survival time after a given cancer diagnosis is increasing. Lifestyle factors, including excess body weight and low physical activity levels, are detrimental both to the development of cancer and clinical outcomes upon diagnosis,2-4 and to the development of diabetes and CVD.⁵ These risk factors may persist after cancer treatment. Maintaining a healthy body weight and getting adequate physical activity can reduce the risk of all-cause mortality among cancer survivors;6-8 however, population-based surveys indicate that cancer survivors generally fare worse in meeting weight and physical activity recommendations than the rest of the population.9 In consideration of this, there is a need for lifestyle programmes that support healthy behaviours and the unique health needs of cancer survivors. The purpose of this study was to test the feasibility and appropriateness of a curriculum developed from the USA Diabetes Prevention Program (DPP)10,11 for individuals previously treated for breast or colon cancer.
METHODS
Study Population
Individuals aged 50–79 years who had a diagnosis of breast (ductal carcinoma in situ, Stages I–III) or colon (Stages I–III) cancer in the prior 60 months were invited to participate in the study. Individuals who completed primary treatments (surgery, radiation, or chemotherapy), did not report a personal history of Type 1 or Type 2 diabetes mellitus (T1/T2DM), had a BMI >25 kg/m², and who self-reported one additional risk factor for T2DM or CVD were eligible to enrol. These additional self-reported risk factors included elevated blood glucose or prediabetes, hypertension, high blood pressure, or taking medications to control blood pressure; dyslipidaemia (high total cholesterol, low high-density lipoprotein cholesterol) or taking medications to control cholesterol; or a family history of T1/T2DM in parents or siblings. The study protocol was approved by the University of Pittsburgh Human Research Protection Office (Institutional Review Board), Pittsburgh, Pennsylvania, USA.
Recruitment
Individuals were recruited between April and June 2016 through a university-sponsored research registry and through the clinics of five medical oncologists who practice within the university hospital system. Information about the study was disseminated to 322 members of the research registry via newsletter and advertised on the research registry online portal. Potential participants contacted by newsletter were those who had indicated a history of cancer (any type) and an interest in weight loss, physical activity, lifestyle, or health and wellness-related research upon enrolling in the registry. Oncologists were approached by study staff and agreed to assist with patient recruitment. Potentially eligible patients were identified by a third-party honest broker who used cancer registry data to identify patients who fit the inclusion criteria and were treated by one of the collaborating oncologists. The identified patients were then mailed a letter with information about the study and signed by the oncologist. In total, 301 letters were mailed to individuals who met the criteria. The oncologists also had clinic staff pre-screen patients who were attending clinic for potentially eligible participants. However, this method was discontinued because undergoing active treatment and thus ineligible for the study.
Eligibility Screening and Enrolment
The target enrolment was 20 participants over a 3-month period. The enrolment target was selected to allow for at least two groups of 8–12 individuals and to minimise the wait time between eligibility screening and the start of lifestyle sessions. Individuals interested in the study were asked to contact the principal investigator by phone to complete a screening interview, following verbal consent. Eligibility screening included questions about cancer diagnosis (month and year, tumour site, and tumour stage) and current diabetes status. If participants met the inclusion criteria for cancer history and diabetes status, they were asked questions about additional T2DM and CVD risk factors, as described above. Participants were provided further information on enrolment if eligible.
Assessment Visits
Eligible participants were invited to attend two assessments, one before and one after the lifestyle programme, scheduled in the cancer centre’s outpatient clinic. At the pre-intervention assessment, written informed consent was obtained. At both assessment visits, participants were asked to complete physical, behavioural, and psychosocial measurements and to provide a fasting blood and urine sample. Blood collection was completed by a registered nurse into 3–10 mL tubes, for a total collection of 30 mL at each visit. Participants were provided with a 90–120 mL urine collection cup and asked to provide a specimen in a private bathroom during a single void. Blood and urine samples were processed immediately by qualified staff and stored in the cancer centre biobank for future analysis.
A trained study staff member performed physical measurements which included weight, height, and waist circumference. Participants’ weight was measured in light clothing and without shoes to the nearest 0.1 lb on a digital scale. Participants’ waist circumference was measured at the midpoint between the iliac crest and the bottom of the 12th rib to the nearest 0.250 inch using a disposable tape measure. Participants’ height was measured without shoes to the nearest 0.125 inch using a stadiometer. Each measurement was repeated once to improve accuracy and averaged for analyses. BMI was calculated from measured weight and height.
Physical activity was assessed by an interviewer-administered questionnaire modified from the 2010 National Health and Nutrition Examination Survey (NHANES).12 The NHANES questionnaire is derived from the Global Physical Activity Questionnaire (GPAQ), which is considered a valid and reliable instrument for capturing moderate and vigorous physical activity.13 The NHANES questionnaire captures time spent in vigorous and moderate intensity activity as part of work and recreation in a typical week. The sedentary behaviour section was modified for this study to individually query time spent in sitting activities in a typical day for occupation, transportation, leisure screen-time (television and computer), and other leisure (e.g., reading, playing cards, and socialising). The questionnaire was scored using the available codebook and algorithms from NHANES.12
Psychosocial measurements included fatigue and quality of life elements. Fatigue was captured by the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale.14 The FACIT-F includes 13 Likert scale items that assess fatigue symptoms over the past 7 days. Item responses range from ‘not at all’ to ‘very much’ (0–4). For scoring, positive responses are given a higher value and a higher score indicates less fatigue. Quality of life was captured by the SF-12® version 1.15 The SF-12 includes 10 Likert scale questions and 2 ‘yes or no’ response questions. Available scoring algorithms were used to calculate an overall score and to calculate the physical and mental composite subscales. A higher score signifies a better quality of life.
Intervention
The DPP-Group Lifestyle Balance (DPP-GLB) is a modified DPP curriculum developed at the University of Pittsburgh for use in community settings16 and is available online.17 The main modifications from the original DPP include: 1) group-based sessions rather than individual; 2) condensation of core material from 16 to 12 sessions; and 3) the addition of post-core maintenance sessions to support participants in sustaining weight and physical activity changes. The DPP-GLB topics include healthy eating,
physical activity, planning, and problem solving. For this pilot, the first 12-weekly DPP-GLB sessions plus 1 session on resistance exercises were offered, beginning in August 2016 and concluding in October 2016. The introduction in Session one was modified to present information on the link between diabetes risk factors and cancer development and outcomes. Consistent with the DPP,10,11 participants were given calorie, fat, and physical activity goals to facilitate a 5–7% weight loss. Calorie (1,200–2,000 kcal per day) and fat (33–50 g per day) goals were determined from initial body weight and could be adjusted based on safe, progressive weight loss of 1–2 lb per week. The physical activity goal was a minimum of 150 minutes per week of moderate activity, similar in intensity to a brisk walk, consistent with recommendations for those with a history of cancer.⁷ Physical activity goals began at 60 minutes per week in Session 4 and progressed to the minimum goal of 150 minutes per week over the remaining 8 weeks of the programme. For participants with higher starting activity levels, their goals were adjusted to meet desired weight loss and lifestyle change. Participants were asked to track their daily weight, calorie and fat intake, and physical activity during the programme in either a paper diary that was supplied by the programme or in online or mobile applications of their choice. The programme was offered in two groups; each session was held both in the morning and evening to accommodate a variety of schedules. Each group had 8–12 participants, with some flexibility to allow participants who could not attend their regular group meeting to attend the other group. Sessions were held in classroom-style conference rooms at the university’s cancer centre and each session was 1 hour. Additionally, parking vouchers were offered to reduce burden on participants and improve attendance. The lifestyle coach was an exercise physiologist and public health professional who received training to deliver the DPP-GLB programme from the University of Pittsburgh Diabetes Prevention Support Center.
Outcomes
The primary outcomes were feasibility metrics as suggested by the National Center for Complementary and Integrative Health (NCCIH).18 The study investigators collected information on the number of individuals reached, screened, eligible, and enrolled; and the costs associated with recruitment and programme delivery. The costs associated with research activities were not included in the feasibility metrics since these costs would not be applicable to community-based delivery of diabetes prevention programmes. Once enrolled, investigators collected participant attendance and adherence to recommended healthy lifestyle behaviours. Attendance was recorded in a weekly log by the lifestyle coach. In-person, phone, or email contact to deliver and discuss the session materials was documented as attending a session. Dietary and physical activity records in self-monitoring books were evaluated for adherence to the weekly dietary and physical activity goals. Participant feedback about the programme features they liked best and least and suggestions for improvement were collected at the end of the study. Completion of the study assessment visits was also considered as a feasibility measure. The study investigators appraised the time required for the assessment visits and the willingness of participants to complete the research measures, including providing a blood and urine sample to be banked for future biomarker analysis (not performed as part of this study). Secondary outcomes included weight, waist circumference, physical activity and sedentary behaviour, and psychosocial measures.
Statistical Analyses
Descriptive statistics were used to summarise feasibility metrics, participant characteristics, and baseline measurements. Paired-samples t-tests and Wilcoxon rank sum tests were used to evaluate changes in secondary outcomes. Statistical significance was not evaluated as this was not the aim of this pilot work and is not appropriate.18
RESULTS
Recruitment and Enrolment
Study information was directly sent to 623 potential participants through the research registry and mailed letters (Figure 1). A total of 44 individuals responded to study advertisements or recruitment letters and were screened for eligibility in the study. Of those screened, 23 (52.3%) were eligible, and 17 (73.9%) of those who were eligible were subsequently enrolled in the study.
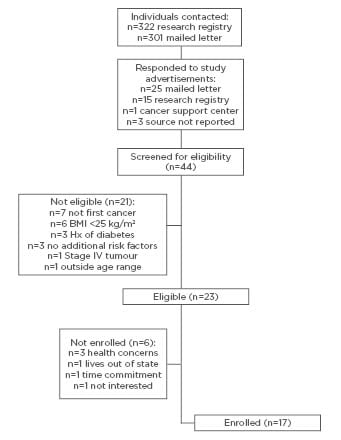
Figure 1: Recruitment, enrolment, and follow-up of participants in a diabetes prevention programme for cancer survivors.
The flow of participants and reasons for ineligibility and not enrolling, respectively, are depicted in Figure 1. At the end of the recruitment period, 85.0% of the target enrolment was reached. According to the interviewer-administered questionnaire at the baseline assessment visit, the mean age of enrolled participants was 60.1 (standard deviation [SD]: ±7.8) years. The mean time since diagnosis was 554.4 (SD: ±516.2) days (approximately 18.5 months). Participants were predominantly women (94.1%) and ethnicity was identified as white (70.6%) or black (29.4%). Participants’ educational attainment was 5.9% high school diploma, 41.2% associate degree, 23.5% bachelor degree, and 29.4% graduate degrees. Most participants were either working full-time (52.9%) or were retired (23.5%). Participants had mostly been treated for breast cancer (94.1%) and had Stage I (41.2%) or Stage II (29.4%) cancer.
Costs for Recruitment and Programme Delivery
The total cost for printing and direct mailing to potential participants was $175 ($30 for printing, $145 for postage at $0.48 per piece). The contact with participants through the online research registry had no direct measurable costs. Recruitment costs were approximately $10 per enrolled participant.
The materials (approximate costs) for the DPP-GLB delivery included a digital scale ($100), food models ($240), participant handouts ($110), participant binders ($60), keeping track booklets ($165), Calorie King® books ($144), pedometers ($124), resistance bands with door stop anchor ($412), and resistance exercise DVD ($85). The total cost for intervention materials and supplies was $1,440 and the cost per enrolled participant was approximately $85. The wages for the lifestyle coach were not included in the programme delivery costs in this study.
Attendance and Adherence
The median attendance was 10/13 sessions (76.9%) (Figure 2), with 82.4% of the participants completing at least 10 sessions. Of the 17 participants who enrolled, 14 actively engaged in the lifestyle programme via contact with the lifestyle coach either face-to-face at sessions or by phone and email. Three participants discontinued the programme due to work schedule conflicts. Overall, participants demonstrated moderate adherence to self-monitoring and meeting diet and physical activity goals (Figure 2). Median submission of diet records was 10/11 (90.9%) and submission of activity records was 8/8 (100.0%). Participants were able to meet goals for calories a median of 8/11 weeks (72.7%), fat grams a median of 5/11 weeks (45.5%), and goals for physical activity a median of 4.5/8 weeks (56.3%).
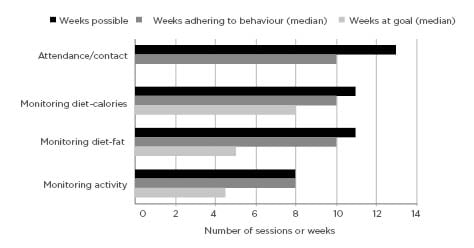
Figure 2: Attendance and adherence to recommended programme behaviours and self-monitoring during participation in a diabetes prevention programme for cancer survivors.
Study Assessment Visits: Physical, Behavioural, and Psychosocial Outcomes
Each study assessment visit required about 1 hour for study questionnaire administration, physical measurement, and specimen collection. The pre-intervention assessment required an additional 15 minutes for informed consent. Overall, participants were willing and able to complete the study assessments. For the main behaviours of weight and physical activity, participants started the programme with an average BMI of 33.1 kg/m2 and moderate levels of physical activity (Table 1). The median weight loss was 4.5% of initial body weight, with a range of 0.4–11.6%. The median physical activity at the end of the programme was 297 min/week. Participants reported increased physical activity, decreased sedentary time, and favourable changes in psychosocial measurements (Table 1).
Participant Satisfaction and Feedback
Overall, participants had a favourable view of the programme, indicating that they liked “the encouragement,” “weekly group sessions with progression of the topics,” and “group interaction.”
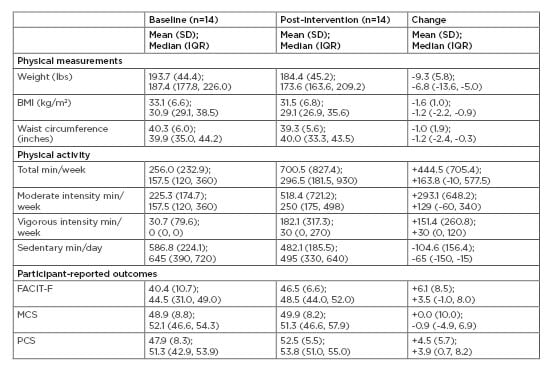
Table 1: Weight, physical activity, fatigue, and quality of life outcomes following a 13-week diabetes prevention programme adapted for cancer survivors.
FACIT: functional assessment of chronic illness therapy; IQR: interquartile range (25th percentile, 75th percentile); MCS: mental component summary; PCS: physical component summary; SD: standard deviation.
Some participants expressed the desire to continue lifestyle sessions and wanted a longer programme.
Feedback for improving the programme included employing a lifestyle interventionist with specialty oncology training to better tailor the programme and answer participant questions. Some participants reported a negative emotional reaction to being at the cancer treatment centre as part of the lifestyle programme.
DISCUSSION
In this feasibility study, individuals with a history of breast or colon cancer were invited to participate in a 13-week lifestyle modification programme. The overall reach was low (44/623 [7.06%] potential participants responded), indicating the need to explore additional strategies for recruitment of cancer survivors to lifestyle programmes. However, once participants enrolled, the DPP-GLB curriculum was feasible to implement as measured by participant attendance and adherence to recommended dietary and physical activity behaviours.
Given the availability of programme training and resources to nurses, dieticians, and health professionals, DPP such as GLB17 and other recognised curricula19 may be suitable for incorporation into cancer survivorship care to address health behaviours as well as diabetes and CVD risk factors. Health professionals, such as nurse oncologists, may play a key role in future efforts that leverage the success of the DPP by identifying patients who may benefit, engaging these patients in the lifestyle programme, and monitoring patient progress in lifestyle change.
The DPP–GLB programme content was well-received and participants were able to make healthy diet and physical activity changes. The weekly programme goals for calories, fat, and physical activity were met 50–75% of the time. Variations in goal achievement between the three outcomes may be related to the notion that lifestyle change is not an ‘all-or-nothing’ approach and barriers to achieving each on a weekly basis may differ (e.g., time required for physical activity and competing commitments). Another possible barrier to increasing physical activity includes long-term side effects of cancer treatment (e.g., fatigue), which should be considered when adapting lifestyle programmes for cancer survivors. Participant adherence to self-monitoring and dietary goal achievement in this study likely contributed to overall weight loss. The amount of weight loss observed is in line with what has been shown in larger scale DPP translations for other high-risk populations.20,21 Furthermore, the observed median weight loss of 4.5% is approximate to that which is recommended for chronic disease risk reduction.22 One prior attempt to translate the DPP to a population of breast cancer survivors23 was feasible and demonstrated similar changes in body weight as those seen in this study. For weight loss, these studies support further testing and evaluation of DPP-based programmes for weight management among cancer survivors. In the present study, participants met the physical activity goal with slightly less frequency than dietary goals. Nevertheless, the results of increased physical activity, decreased sedentary time, and improvements in quality of life metrics here are consistent with other community interventions using the DPP–GLB programme20 and may demonstrate potential for additional cancer survivorship benefit.8,24
The cost of delivering primary and secondary prevention programmes is a concern, especially in the USA where healthcare costs continue to escalate.25 In this study, the estimated costs per participant were about $85. When delivered in the community setting, additional costs may include facility rentals, lifestyle coach wages, and administrative fees which were not accounted for in this study. While this may increase overall costs, partnerships within the community and use of existing space and resources can help minimise these costs. Also, for DPP–GLB programme delivery, some items that were purchased such as a digital scale and food models may be used in subsequent programme delivery and thus reduce future costs. In the current delivery model, providers or participants are responsible for the costs of prevention programmes. The Centers for Medicare & Medicaid Services (CMS) has led the way in reimbursement for participation in US Centers for Disease Control and Prevention (CDC) recognised DPP programmes.19,26 Other public and private insurers are following in these footsteps. This is anticipated to ease the burden of cost from the provider and participant perspective, while supporting the belief that dollars spent on prevention will pay dividends in future averted medical costs related to diabetes and obesity.
Although the lifestyle programme was delivered with high retention and participant satisfaction, there were limitations to the research. First, enrolment fell short of the modest target of 20 participants. This may be because of the short time frame (3 months) that the study team attempted to recruit or the effectiveness of the approaches (i.e., research registry and mailed letters). Second, there were few potential participants who contacted the study team who had a history of colon cancer, resulting in 94.1% of participants having a history of breast cancer. Alternate strategies, such as peer-led recruitment, may be needed to engage those with a history of colon cancer in lifestyle programmes.27 Thus, the findings from this study may not extend to individuals with a history of other cancers. Third, self-reported participant risk factors for diabetes or CVD may lead to misclassification of actual diabetes or CVD risk at time of study enrolment. The results for secondary outcomes should be interpreted cautiously, because hypothesis testing and determination of statistically significant changes in the outcomes was not the focus of this pilot research. Lastly, healthy volunteer bias may result in a participant sample with fewer complications related to cancer treatment and thus limit the generalisability of the findings.
CONCLUSION
Overall, this article supports larger efforts to evaluate use of DPP curricula for breast cancer survivors with risk factors for T2DM and CVD.
The results of this study suggest additional approaches may be needed to recruit cancer survivors into diabetes prevention programmes. However, once enrolled, this pilot demonstrated feasibility in achieving participant engagement and adherence to the lifestyle goals. Leveraging successful prevention programmes like the DPP into new fields, i.e., cancer survivorship, has the potential to improve health behaviours and chronic disease risk factors in an increasing portion of the ageing population.








