Abstract
Diabetes is known to be a risk factor for active pulmonary tuberculosis (TB) and the reactivation of latent or previous TB. It is also associated with poor TB treatment outcomes. Conversely, TB infection in itself can worsen glycaemic control temporarily and possibly lead to diabetes, among other non-communicable comorbidities. Post-TB lung disease decreases life expectancy and increases the risk of recurrent TB infection. There are efforts in low- and middle-income countries to integrate TB and diabetes healthcare services, as encouraged by the WHO and other international health organisations. However, integration measures, including bidirectional screening and coordinated care for both diseases in low- and middle-income countries, are scarce. This may lead to a lack of control over either condition. The authors present the journey of a 48-year-old man with Type 2 diabetes and previous pulmonary TB. He presented with a 2-week history of productive coughing and massive haemoptysis amounting to 500 mL in total. Recurrent pulmonary TB was confirmed by Gene Xpert, a chest X-ray, and CT of the chest. Glycaemic control improved while on TB treatment after counselling on adherence. The difficulties and dilemmas in managing and following up on a communicable and non-communicable disease traditionally cared for can be improved upon with the integration of TB/diabetes healthcare services.
Key Points
1. Erosion of blood vessels in the pulmonary circulation and intercostal arteries leads to massive haemoptysis, which accounts for 5% of tuberculosis deaths before effective treatment.
2. This is a case report of recurrent tuberculosis and severe haemoptysis in a patient with dysregulated diabetes.
3. Integrating healthcare services can improve management and follow-up for communicable and non-communicable diseases, such as tuberculosis/diabetes.
INTRODUCTION
People with diabetes have a threefold increased risk of developing active tuberculosis (TB) compared to those without diabetes,1,2 leading to treatment failure, relapse, and death.1 Both Type 1 diabetes and Type 2 diabetes are risk factors for the development of active TB, with Type 2 diabetes accounting for the majority of TB cases.3 Research has shown that Type 2 diabetes patients have innate and adaptive immune response dysfunction, such with complement system, dendritic cells, and macrophages, which contribute to the development of opportunistic infections such as TB.4 It is therefore becoming increasingly important that early detection and rigorous management of both diabetes and TB be done and integrated.5 Pulmonary TB in patients with diabetes presents with multiple complications such as pneumothorax, bronchiectasis, fistula, pulmonary gangrene, chronic pulmonary aspergillosis, tracheobronchial stenosis, and malignancy. Massive haemoptysis is one of the complications the authors’ patient presented with and occurs due to the erosion of blood vessels in the pulmonary circulation and intercostal arteries. This is responsible for 5% of TB deaths before effective treatment.6
In a meta-analysis conducted in 2018, the pooled prevalence of TB among patients with diabetes in Africa was 5.13% (95% CI: 4.34–5.92) due to TB and diabetes comorbidity.7 However, in 2017, Peer et al.8 stated that diabetes prevalence in Africa might be underestimated as between 50.7–75.1% are undiagnosed, especially in low- and middle-income countries (LMIC). According to reports, Tanzania has a high TB endemic, with 293 cases per 100,000 people between the ages of 15–64 years, and 709 cases per 100,000 people over 65 years.9
The WHO, the International Union against Tuberculosis and Lung Diseases, and the International Diabetes Federation (IDF) recommend an integrated approach to TB/diabetes health care due to the higher risk of developing TB in patients with diabetes as well as the difficulties faced by TB patients with diabetes in the treatment and care of both diseases.10,11 As a result, integration aims to replicate strong infectious disease outcomes for patients with other chronic conditions, improve the efficiency and quality of the service, and facilitate access to care for patients with non-communicable diseases (NCD) while maximising efficiency in light of the acute shortage of human resources.12 Integration of TB and diabetes health care is defined as a variety of managerial or operational changes to health systems to bring together inputs, delivery, management, and organisation of TB and diabetes service functions, as well as different kinds of diabetes and TB services, operational programmes, or activities joined together to ensure and perhaps maximise collective outcomes for both patients with diabetes and with TB.13
In response to the limited efforts on TB/diabetes integrated healthcare services, especially in the LMICs, the Adaptive Disease control Expert Programme in Tanzania (ADEPT) is supporting the integration of communicable and NCDs using TB and diabetes as a case study.14 This support included appropriate clinical management, including for those with HIV co-infection, training of healthcare providers on bidirectional screening of TB and diabetes, and the application of glycated haemoglobin (HbA1c) for guiding the selection of hypoglycaemic agents in routine settings in Tanzania.14 Evidence of the true impact of integrated NCD/TB programmes on patients is still lacking in Tanzania, despite the efforts made in the integration process. The authors present a case demonstrating TB/diabetes service disintegration in Tanzania, which resulted in recurrent TB infection.
CASE DESCRIPTION
A 48-year-old man presented with a 2-day history of coughing blood, which was fresh blood mixed with clots. He had about three episodes, which amounted to about 500 mL of blood in total. There was a positive history of fevers, night sweats, and significant weight loss. He has longstanding, poorly controlled Type 2 diabetes with a recent history of painful peripheral neuropathy (PNP). He has been living with diabetes for 5 years on oral-hypoglycaemic agents, namely metformin and glibenclamide, with no clear records of self-blood glucose monitoring at home. Previously, he was treated for TB in 2021 with four tablets of rifampicin 150 mg/ isoniazid 75 mg/ pyrazinamide 400 mg/ ethambutol 275 mg (RHZE) for 2 months, followed by four tablets of RH for 4 months, and was declared cured. He is a butcher who does not smoke cigarettes or drink alcohol.
On examination, he was an ill-looking man with a BMI of 18.3 kg/m2 and dyspnoea with a respiratory rate of 32 breaths/min on oxygen (12 L) via a non-rebreather face mask with an oxygen saturation of 95%. He was not pale, not jaundiced, not cyanosed, and had no palpable peripheral lymphadenopathy. He had a temperature of 37.1 °C, a blood pressure of 116/81 mmHg, a heart rate of 91 bpm, and a random blood glucose of 19.3 mmol/L. On respiratory examination, he had symmetrical chest expansion with diffuse bilateral crackles, and the rest of the systemic examination had insignificant findings.
Initial investigations revealed an erythrocyte sedimentation rate of 120 mm/h; a full blood picture revealed a leucocyte count of 6.76×109 /L, haemoglobin of 13.3 g/dL, and platelets of 132×109 /L. The bleeding indices were normal, with activated partial thromboplastin time of 34 seconds and an international normalised ratio of 0.9. Baseline HbA1c was 143 mmol/mol. His biochemical investigations were within normal ranges. A urinalysis revealed significant glucose and protein present in the urine. A sputum gram stain revealed candida species with gram positive cocci. A sputum gene-Xpert confirmed TB with no resistance. A serology for HIV was negative.
A chest X-ray (Figure 1) was suggestive of pulmonary TB with fibrosis in the apical segment of the right upper lobe and a thick-walled cavity in the left midzone. A CT scan of the chest revealed fibro-cavitary lesions in the apical segment of the right upper lobe and the superior segment of the right lower lobe (Figure 2). Additionally, scattered nodules were seen in the left upper lobe, suggesting post-primary tuberculosis.
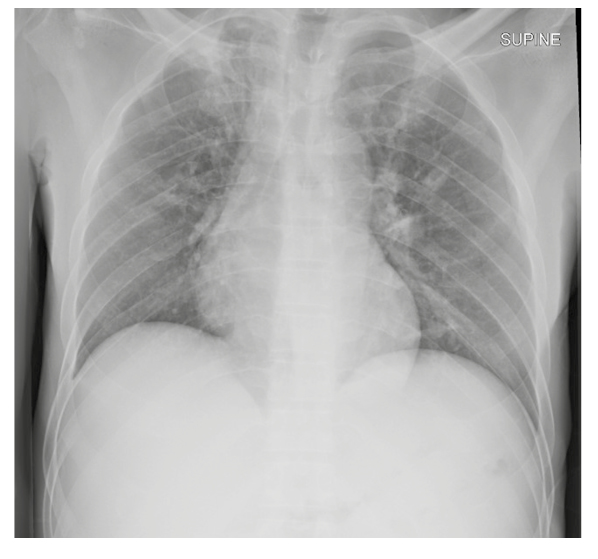
Figure 1: A chest X-ray showing fibrosis in the apical segment of the right upper lobe and a thick-walled cavity in the left midzone.
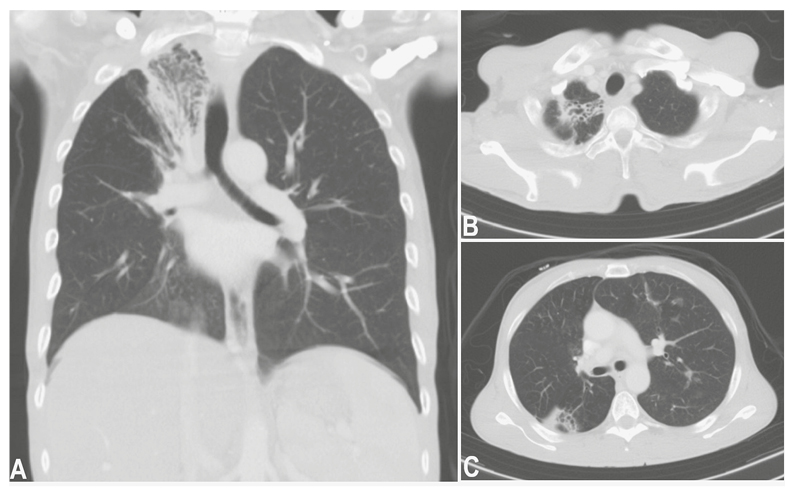
Figure 2: Chest CT, axial and coronal views, demonstrated fibro-cavitary lesions in the apical segment of the right upper lobe and the superior segment of the right lower lobe.
Scattered nodules seen in the left upper lobe suggests post primary tuberculosis.
According to the Tanzanian standard treatment guidelines, the patient was started on four tablets of RHZE for the initiation phase and pyridoxine 100 mg once daily due to suspected vitamin B6 deficiency as a result of anti-tuberculous medications he had previously used. During the course of treatment, the patient was reluctant to start insulin injections, despite his poor glycaemic control. He was counselled on starting insulin injections but refused. Thus, counselling continued as he continued with oral hypoglycaemic agents.
During the 1-month follow-up clinic, he was still taking RHZE and oral hypoglycaemic medications with very poor glycaemic control, with random blood glucose up to 17 mmol/L on oral hypoglycaemic agents. After multiple sessions of counselling, the patient was initiated on insulin, and the follow-up of both diabetes management and TB treatment was done at one clinic. There was much improvement after 3 months, as the fasting blood glucose levels ranged from 7–11.5 mmol/L, the follow-up HbA1c was 110 mmol/mol, and BMI had increased to 20.1 kg/m2 after extensive sessions with the nutritionist about his diabetic diet and TB supplementation diet. He was then switched to four tablets of RH, which were prescribed for the next 4 months. The timeline of the events is shown in Table 1.
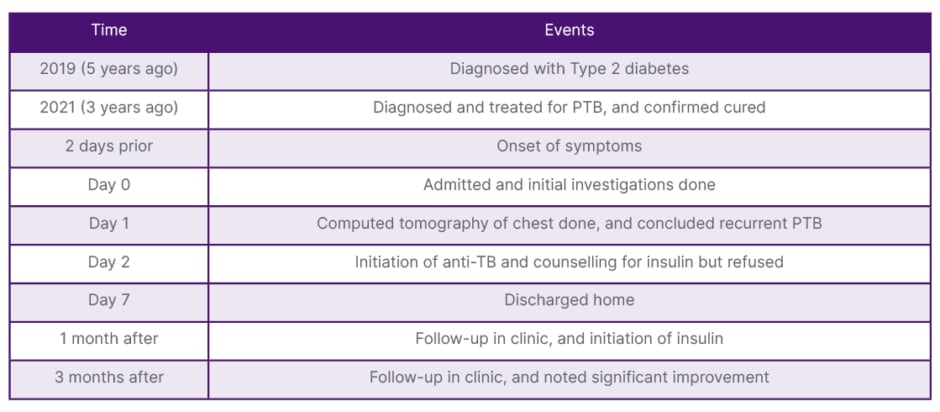
Table 1: Timeline of events.
PTB: pulmonary tuberculosis; TB: tuberculosis.
DISCUSSION
This case report illustrates the challenges an individual has in controlling diabetes, thus resulting in a recurrent TB diagnosis with a potentially life-threatening complication. It is well established that poorly controlled diabetes increases the likelihood of unfavourable TB outcomes, such as treatment failure, relapse, and death. Furthermore, after receiving TB treatment, patients with diabetes may continue to have positive sputum smear results for AFB for 2–3 months.15 In another study, 22% of patients with diabetes and TB remained sputum culture positive after a 6-month treatment course with TB medications.16 Consequently, in order to improve the course of their TB therapy and avoid complications from poorly controlled diabetes, these patients require special attention and care.
Having treated this patient with massive haemoptysis, apart from TB, the possible differential diagnosis included bronchiectasis, mycetoma, necrotising pneumonia, cryptogenic haemoptysis, and bronchogenic carcinoma.
Recent literature shows that TB causes fewer massive haemoptysis cases but still remains the most common cause with high prevalence in Africa and China.17
The integration of diabetes and TB services in Malawi has been well described and categorised under the complex model, which includes HIV and TB services in addition to NCDs, all provided by the same healthcare professionals in a single clinic.12 Given the rising incidence of TB and diabetes comorbidity worldwide, integrated approaches to care have been suggested as a means of effectively managing both conditions, particularly in LMICs where TB is widespread, 80% of all cases of Type 2 diabetes occur, and health systems are underdeveloped.18 With regards to treatment outcomes, integrated care had greater TB treatment success than traditional non-integrated care, even after adjusting for age and sex. It also has less treatment loss to follow-up than non-integration. Especially in TB-endemic regions, integrated care services may lessen the chance of unfavourable treatment outcomes for patients with comorbidity than for those with TB alone.19 Although a study in India has mentioned protocols for administrative set-up and training for TB/diabetes integration, they have noted challenges, particularly in primary-level research, monitoring, and evaluation. The challenges were identified in strengthening the individual TB and diabetes programmes and implementing an integrated TB/diabetes healthcare system.20
Several studies have reported that diabetes is an important predictor of unfavourable TB treatment outcomes.21 A systematic review concluded that optimal glycaemic control is vital for improving treatment outcomes, as well as reducing susceptibility and minimising complications. The emphasis on effective healthcare strategies and management is crucial in achieving control.22 But a study done in Tanzania found that impaired glucose regulation did not influence TB treatment outcomes such as treatment success, microbiological clearance, or death.19 However, compared to a non-integrated care system, collaborative management of TB and diabetes comorbid patients reported decreased proportions of treatment loss to follow-up and increased treatment success, according to an implementation research study done in Mexico.23
Retention in care has been linked to early diagnosis and glucose monitoring, according to studies. In 2018, Harries et al.3 discovered that poor glucose control, underdiagnosis, inadequate treatment, and inadequate glucose monitoring seem to pose a much bigger threat to TB care and prevention than previously thought. The authors also noted that uncontrolled glucose among patients wih diabetes is a critical issue in LMICs.
If TB and diabetes integrated care services are to be scaled up within hospitals in Tanzania, lessons from piloting hospitals in other countries should be considered. The initiative process has been done through the ADEPT programme model in Tanzania.14 In order to facilitate early bidirectional screening and co-management of TB and diabetes, along with other related comorbidities, the model includes a mentorship package for frontline healthcare workers. In addition, the programme provided teaching materials, instruction/algorithms, and glucometers with glucostrips to health facilities to aid in the process. A clinical audit was also implemented as a way to direct changes and enhancements to the clinical standards for the management of multiple morbidities.14 The integration of TB/diabetes programmes could contribute to health systems strengthening, and ensure patient-centeredness and continuum of care.24 Thus, considerations should be made on the actual activities to integrate and the level of integration within the health system, and this will go hand in hand with the efficiency of service delivery and monitoring. Research has shown that the existence of national guidelines ensures the availability and readiness of integrated services.24
CONCLUSION
The present case of recurrent TB in a patient with haemoptysis in uncontrolled diabetes underscores the need for service integration in Tanzania and other countries with a high dual burden of TB and diabetes. The burden of the disease, the currently weak evidence of an impact on bidirectional screening coverage and treatment outcomes of both diseases, the obstacles encountered in the local context, and the guidelines already in place should all be carefully considered when deciding whether or not to integrate TB/diabetes healthcare services in hospitals.







