Abstract
Diabetes is a chronic condition that afflicts over 450 million people worldwide. Diabetes can lead to the development of multiple chronic comorbidities, such as microvascular, macrovascular, and neuropathic complications. Furthermore, diabetes is the leading cause for many of these complications, such as blindness, peripheral arterial disease, and kidney disease. Many of these conditions can go unnoticed for many years until they become more severe and are no longer reversible. This article will provide an evidence-based review of the background, prevention, and screening for many of the complications of diabetes.
INTRODUCTION
Diabetes is a condition characterised by either defects in insulin secretion, insulin sensitivity/action, or both. These include Type 1 diabetes (T1D), Type 2 diabetes (T2D), gestational diabetes, and diabetes secondary to other medical conditions. Diabetes is one of the fastest growing global emergencies of the 21st century. Based on the International Diabetes Federation (IDF) Diabetes Atlas 2021,1 it is estimated that 537 million people have diabetes; this number is expected to reach 643 million by 2030 and 783 million by 2045. In addition, at the time of writing, 541 million people are estimated to have impaired glucose tolerance in 2021. It is also estimated that over 6.7 million people aged 20–79 years will have died from diabetes-related causes in 2021. Early diagnosis is important to prevent the complications associated with diabetes. In one study of 200 newly diagnosed patients, 52%, 10%, and 6% of patients were found to already have signs of neuropathy, nephropathy, and retinopathy, respectively.2 In another study, patients with insulin-dependent diabetes had a 27.2% and 53.5% prevalence of macro- and microvascular complications, respectively.3 This review discusses many of the complications associated with diabetes, as well as the approach to screening.
MACROVASCULAR DISEASE
Macrovascular disease in diabetes is due to atherosclerosis, which leads to myocardial infarction, stroke, and peripheral arterial disease. Macrovascular disease is the leading cause of death for adults with T2D. Diabetes is a proinflammatory and thrombotic state that is associated with endothelial damage. High glucose also leads to an imbalance of nitric oxide bioavailability and reactive oxygen species, which leads to endothelial dysfunction. This is further compounded by the elevated low-density lipoprotein particles that accumulate in the endothelial walls. The American Heart Association (AHA) and the American Diabetes Association (ADA) have recommendations for primary prevention of cardiovascular disease (CVD) in patients with diabetes (Table 1),4 yet only half of adults with T2D are adhering to some of these recommended guidelines, such as management of dyslipidaemia.5 Furthermore, atherosclerotic disease is responsible for 42% of mortality in diabetes.6 It is important to understand the risks for these conditions, appropriately test and screen for them, and initiate primary and secondary prevention.
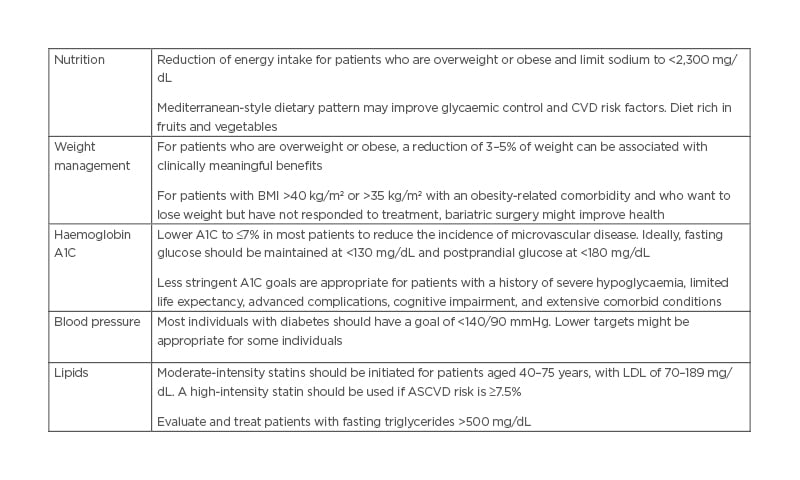
Table 1: The American Heart Association (AHA)/American Diabetes Association (ADA) guidelines for cardiovascular disease risk factor.
ASCVD: atherosclerotic cardiovascular disease; A1C: glycated haemoglobin; CVD: cardiovascular disease; LDL: low-density lipoprotein.
Peripheral Arterial Disease
Background
Peripheral arterial disease (PAD) is present in approximately 29% of patients with diabetes and may be underdiagnosed due to confounders, such as a lack of sensation from neuropathy to identify pain.7 However, patients with PAD and diabetes are more likely to have symptoms of claudication, and more likely to present with ulcers, increasing the risk for limb amputation.8
Screening and prevention
It is recommended that as part of a thorough history, patients with diabetes are asked about limb claudication as well as foot ulceration. Furthermore, all patients should have inspection of feet and lower extremity vasculature, including femoral, popliteal, posterior tibialis, and dorsalis pedis pulses. The ADA recommends screening all patients with diabetes over the age of 50 years with Ankle Brachial Index (ABI) measurements and, if normal, repeating in 5 years.9
Coronary Heart Disease
Background
T2D is considered a coronary heart disease (CHD) equivalent, since patients with T2D without known CHD have the same risk of myocardial infarction and death from CHD as those with a prior history of myocardial infarction.10 Almost 75% of patients with diabetes are found to have obstructive or non-obstructive coronary artery disease on a CT angiogram.11
Screening and prevention
While diabetes does lead to a significantly elevated risk, it is not recommended to screen patients without symptoms, as the evidence has failed to show improved outcomes with this approach. It is recommended that patients have risk factors aggressively treated as those with a previous history of known coronary artery disease. Patients should be screened annually for being overweight or obese, hypertension, dyslipidaemia, tobacco use, chronic kidney disease (CKD) or albuminuria, and family history of CHD. If any are present, then they should be managed appropriately.12
Hypertension
In addition to lifestyle modification, intensive treatment of hypertension with a goal blood pressure of <140/90 mmHg for those with lower cardiovascular (CV) risks, and <130/80 for those with higher CV risks (10-year atherosclerotic CVD [ASCVD] risk of ≥15%) should be initiated. Initial treatment should be with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) if albuminuria is present.
Lipid management
In addition to lifestyle modification, patients aged 40–75 years should be started on a statin. General recommendations are that in those without CVD, use of a moderate-intensity statin is indicated. For those with multiple risk factors, known CVD, or aged 50–70 years, a high-intensity statin is indicated. In those with ASCVD risk of ≥20%, it would be reasonable to add ezetimibe to statin therapy.
Stroke
Background
Just as diabetes increases the risk of CHD, the risk for stroke is also increased, with a relative risk of 2.0–5.8, with risks being higher in females than males. In addition, patients with T2D have a higher proportion of ischaemic stroke versus haemorrhagic stroke compared with the general population.13
Screening and prevention
There are no current guidelines for screening for carotid atherosclerosis in patients with diabetes. However, in patients with other occlusive disease or CV risk factors, there may be benefits.14 In regard to prevention, the recommendations follow the same listed above for managing other CV risk factors, such as hypertension and lipid management. Patients should also have tight glucose control, although the evidence is not as robust as for microvascular complications.
Aspirin in Diabetes
For primary prevention of CVD, the ADA recommends that patients with diabetes and an ASCVD risk of >10% or with known CHD should be placed on aspirin 75–162 mg daily, after discussing the risks of increased bleeding with the benefit of reduction in CVD. For patients with ASCVD risk of <5%, the increased risk of bleeding likely outweighs the benefits. For patients with ASCVD risk of 5–10%, shared decision making can help decide whether aspirin would be indicated.12
MICROVASCULAR COMPLICATIONS
Microvascular complications in diabetes include neuropathy, retinopathy, and nephropathy. Below is a review of each of these complications.
Neuropathy
In addition to vascular disease, patients with diabetes are also afflicted by neuropathies. The two most common neuropathies include distal symmetric polyneuropathy (DSPN) and CV autonomic neuropathy (CAN), affecting 19% and >50% of Americans with diabetes, respectively.15,16 Guidelines for prevention of all neuropathies include optimising blood glucose levels as early as possible to prevent or slow the development of both DSPN and CAN.17
Distal symmetric polyneuropathy
DSPN is defined as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after exclusion of other causes.”17 This is thought to be caused by oxidative and inflammatory stress, which leads to damage of the nerve cells. It is recommended that all patients with T2D be screened annually for DSPN, and those patients with T1D should be screened annually 5 years after initial diagnosis. Screening is performed by taking a thorough history and examination. The history may indicate a feeling of pressure or feeling off-balance. Patients may also describe numbness and tingling, and occasionally pain. The physical exam should include assessment of either temperature or pin prick sensation, and vibration sense using a 128 Hz tuning fork. In addition to these assessments, patients should have a 10 g monofilament test to assess for the risk of ulceration. Patients should be educated on the risks of neuropathy and PAD and check their feet regularly. If patients do have DSPN, approved medications include pregabalin and duloxetine. However, other medications such as tricyclic antidepressants and some opioids can also be used.17
Cardiovascular autonomic neuropathy
CAN leads to symptoms of hypoglycaemia unawareness, orthostatic hypotension, gastroparesis, bowel changes including faecal incontinence, erectile dysfunction, and other autonomic dysregulations. While CAN is not prevalent in early diabetes, its prevalence increases with time, with 60% of patients with T2D having symptoms consistent with CAN after 15 years.17 Furthermore, CAN is an independent risk factor for increased mortality.18,19 The most common symptoms patients have are light-headedness, faintness, palpitations, or syncope. These questions should be asked in patients with microvascular and neuropathic complications. In this setting, other causes of those symptoms should be assessed, such as drug interactions.
Variability in heart rate when standing can be indicative of CAN. However, it is typically not identified until later, when symptoms of orthostasis and resting tachycardia present. As above, treatment is with glycaemic control. Symptoms of orthostasis can be treated with midodrine.17
Other neuropathies
Patients with neuropathy should also be screened for gastroparesis by asking about nausea, early satiety, or unexpected glycaemic variability. Both males and females with other forms of neuropathy should be checked for lower urinary tract symptoms such as incontinence and bladder dysfunction. Female sexual dysfunction, including decreased libido, dyspareunia, and inadequate lubrication, should also be screened. Lastly, patients should be screened for erectile dysfunction, including libido and ability to reach and maintain erection.17
Diabetic Retinopathy
Background
Diabetic retinopathy (DR) is one of the main causes of vision loss worldwide and is the main cause of blindness in working age adults in the USA. Over 25% of patients with diabetes will have some form of DR.20 The majority of patients who develop DR have no symptoms until the extremely late stages, making screening asymptomatic patients critical to preserving their vision. The primary risk factors for development and progression of DR are duration and intensity of glycaemic control.21 Uncontrolled hypertension, hyperlipidaemia, and presence of other microvascular complications such as diabetic nephropathy and neuropathy also aid in the worsening of underlying DR.22-24 Controlling these risk factors is important for the prevention of DR.
Screening
Patients with T1D should have their first ophthalmologic examination within 5 years of diagnosis, and those with T2D should have their first examination shortly after diagnosis. If there is no evidence of retinopathy during the screening examination, then ophthalmologic examination should be performed every 2 years. If there are any abnormalities, then the frequency of the exam will need to be yearly or more often, depending on findings.25
Prevention
In patients with diabetes, enhancing glycaemic control and treatment of systemic conditions like hypertension and hyperlipidaemia is essential to prevent vision loss. Because DR occurs exclusively in the setting of hyperglycaemia, good glycaemic control is the key to prevention. Multiple studies have proven that lowering glycated haemoglobin (A1C) reduces the rate and progression of DR.26-28
Blood pressure control
Blood pressure control decreases the incidence of DR and, in some trials, also slows the rate of progression of DR.29 There are not enough data to recommend a specific antihypertensive agent based upon retinopathy endpoints.
Lipid-lowering therapy
The benefit of cholesterol-lowering therapy for the prevention of DR has not been well established. Most patients with T2D will require treatment with statins to control hyperlipidaemia and prevent ASCVD. Lowering triglyceride levels with fenofibrates may have a beneficial effect. As an example, in the ACCORD Eye Study, there was a reduction in progression of retinopathy in patients taking fenofibrate.30
Exercise
Regular exercise and increased physical activity may lead to a reduction in retinopathy.31,32
Diabetic Kidney Disease
CKD is common in patients with both T1D and T2D, occurring in 20–40% of patients with diabetes. Diabetic kidney disease (DKD) is defined by the presence of reduced glomerular filtration rate (GFR) and/or increased urinary albumin excretion for at least 3 months in a patient with diabetes. Globally, DKD is a major cause of CKD, and is the most common cause of end-stage kidney disease.33 In the USA, 48% of patients with diabetes will have microalbuminuria and 8% will have overt macroalbuminuria.34
Background
DKD is a complex and heterogeneous disease, with many overlapping aetiologic pathways, leading to changes in glomerular haemodynamics, oxidative stress, inflammation, interstitial fibrosis, and tubular atrophy. These pathways include activation of the renin–angiotensin system as well as hyperglycaemia.35 While hyperglycaemia undoubtedly plays a leading role, hyperinsulinemia and insulin resistance also may instigate pathogenic mechanisms, possibly explaining the variation in histopathology between T1D and T2D.
Screening
Screening for microalbuminuria (spot urinary albumin-to-creatinine ratio: >30 mg/g creatinine) should be performed annually in patients with T1D of more than 5 years, and in patients with T2D at the time of diagnosis.36 If microalbuminuria is present, non-diabetic conditions causing albuminuria like urinary tract infections, haematuria, heart failure, febrile illnesses, severe hyperglycaemia, and vigorous exercises should be ruled out. Urine tests for albuminuria should be repeated within 3–6 months and treatment should be considered if two of three albuminuria tests are positive.34
Prevention and treatment
Lifestyle modifications like healthy eating, regular exercise, weight loss, and smoking cessation have been recommended to all patients with diabetes, with or without underlying DKD. In addition, because of the increased risk of CVD, patients should also be on statins.
Blood pressure control
Intensive blood pressure lowering has been recommended in patients with DKD as it reduces mortality, prevents CV morbidity, and it may prevent end-stage kidney disease with severely increased albuminuria (urine albumin excretion: ≥300 mg/day).37 Initial antihypertensive therapy in patients with DKD usually consists of ACE inhibitors or ARBs, but not both concurrently.36 It is important to not discontinue use of ACE or ARB if the rise in serum creatinine is less than 30% upon initiation. In patients who will need combination antihypertensive therapy, a calcium channel blocker may be preferred rather than a thiazide diuretic.38
Glycaemic control
Intensive blood glucose control has been known to prevent the development of DKD.39-41 The glycaemic control target in patients with diabetes and DKD is ideally an A1C of 7% or less. The approach to target an A1C of 7% is clear for T1D, but less so in patients with T2D.36 Glycaemic goal should be tailored to achieve a balance of improvement in microvascular complications with the risk of hypoglycaemia.
Sodium–glucose cotransporter-2 inhibitors
Sodium–glucose cotransporter-2 inhibitors reduce the risk of kidney disease progression among patients with DKD who are already taking ACE inhibitors (or ARBs), as well as the incidence of CVD.42 These drugs should be used with caution in patients with a history of lower extremity amputation, ulcers, or peripheral vascular disease who are at risk of future amputation, or in patients with GFR <30 mL/min/1.73 m2.
Monitoring
Patients with DKD should have blood pressure, volume status, GFR, potassium, and A1C evaluated every 3–6 months. In addition, it is important to assess the serum creatinine and potassium within 1–2 weeks of starting or intensifying renin–angiotensin system inhibitor. Patients with advanced CKD (estimated GFR: <30 mL/min/1.73 m2), heavy albuminuria, rapid loss of kidney function, resistant hypertension, or evidence of an inflammatory kidney disease should be referred to a nephrologist.35,36
SUMMARY
Patients with diabetes are at increased risks of macrovascular, microvascular, and neuropathic complications. Based on the DCCT results, patients with T1D who managed to keep their blood glucose levels close to normal with intensive diabetes treatment as early as possible in their disease had fewer diabetes-related health problems after 6.5 years, compared with people who used the conventional treatment. This study also showed that people who used intensive treatment lowered their risk of diabetic eye disease by 76%, DKD by 50%, and diabetic neuropathy by 60%. Participants who used intensive treatment had an average A1C of 7%, while participants who used the conventional treatment had an average A1C of 9%.
Prevention is the most effective way to reduce morbidity and mortality, but screening for these in asymptomatic patients can lead to earlier detection and treatment (Table 2). These guidelines will likely continue to evolve as additional research and studies reveal more effective ways of screening. There are no good screening tests for macrovascular complications of stroke and CHD, and clinicians look at other markers through blood pressure and lipids. While screening for microvascular disease in the form of DR and nephropathy are fairly effective, they have their limitations. Future studies looking into other tests for screening macrovascular complications, as well as more sensitive biomarkers for microvascular complications such as nephropathy, will be advantageous to preventing morbidity in the future.
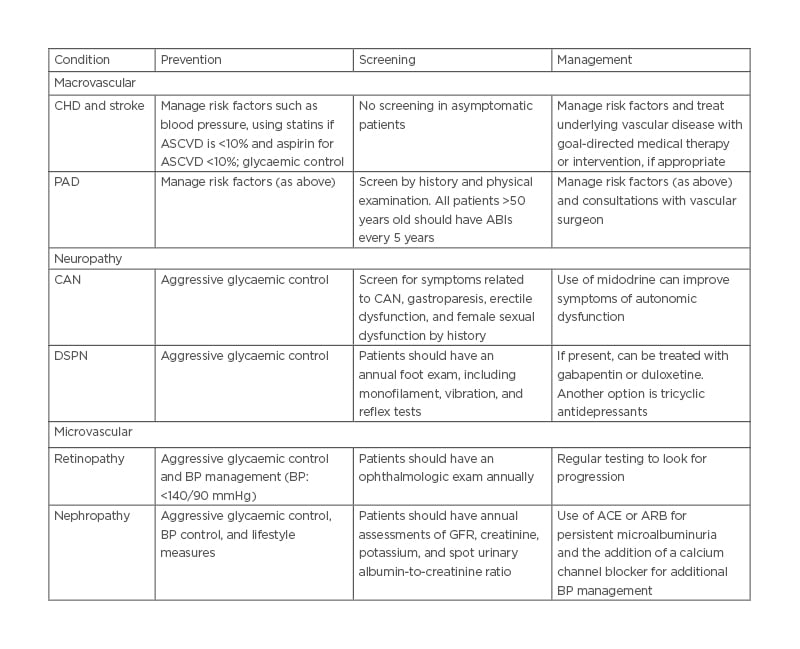
Table 2: Summary of prevention, screening, and management.
ABI: Ankle Brachial Index; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blockers; ASCVD: atherosclerotic cardiovascular disease; BP: blood pressure; CAN: cardiovascular autonomic neuropathy; CHD: coronary heart disease; DSPN: distal symmetric polyneuropathy; GFR: glomerular filtration rate.