Abstract
Ocrelizumab, a novel member of disease-modifying therapies for multiple sclerosis (MS), is a humanised monoclonal antibody against the CD20 molecule on the surface of B cells. Reports on possible effects of this molecule in MS therapy have attracted a lot attention since its approval in 2017.
The authors present a 31-year-old female patient who was diagnosed with MS in 2008, with a concomitant disease of Type 1 diabetes (T1D). The patient’s MS treatment included interferon-β1a and fingolimod prior to ocrelizumab initiation in 2019. In regard to the patient’s T1D course, they had poor glycaemic control despite regular follow-ups and strict treatment plans. Subsequent to the commencement of ocrelizumab therapy, a significant improvement was observed in their glycaemic control.
The authors’ case study aims to raise motivation for further investigation and studies to evaluate this unexpected potential impact of ocrelizumab on T1D control.
Key Points
1. The co-occurance of multiple sclerosis and Type 1 diabetes (T1D) is not uncommon. The former leads to demyelination and neurodegeneration of the central nervous system, while the latter causes the destruction of insulin-secreting β cells in the islets of Langerhans of the pancreas.2. This case study of a 31-year-old female diagnosed with T1D and multiple sclerosis showed that the initiation of ocrelizumab was associated with a significant improvement in glycaemic control within 3 months.
3. As no other studies to date have analysed the mechanism and impact of ocrelizumab in glycaemic control, further studies are needed to further understand its effects on T1D.
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system, causing a plethora of neurological manifestations and a leading cause of non-traumatic disability among young adults. According to MS International Federation data, global MS incidence in 2020 is 75 cases per 100,000.1
MS was initially considered a T lymphocyte-mediated disease. Hence, over the past couple of decades, B lymphocyte involvement has been implicated in MS pathophysiology.2 Therefore, therapies leading to B lymphocyte depletion have been increasingly popular in the treatment of MS. Ocrelizumab is an intravenously administered humanised monoclonal antibody that targets the CD20 marker on B lymphocytes and is approved for the treatment of relapsing–remitting MS and primary progressive MS (PPMS).2 The mechanism by which ocrelizumab works in MS is presumed to involve binding to CD20, a cell surface antigen present on pre-B and mature B lymphocytes. Following cell surface binding to B lymphocytes, ocrelizumab results in antibody-dependent cellular cytolysis and complement-mediated lysis.2
Similar to MS, Type 1 diabetes (T1D) is an organ-specific autoimmune disorder with an inflammatory component, but with marked differences in pathogenesis and clinical manifestations. While MS is associated with demyelination and neurodegeneration of the central nervous system, T1D is responsible for the inflammatory autoimmune destruction of insulin-secreting β cells in islets of Langerhans of the pancreas, which ultimately results in the failure of insulin-mediated regulation of glucose levels. HbA1c can be used as a diagnostic test for T1D. Furthermore, HbA1c concentration is a reliable and commonly used tool for monitoring long-term glycaemic control as well as defining specific treatment targets.3
The authors performed a literature review in databases such as Wiley and PubMed, using the following keywords: “ocrelizumab,” “multiple sclerosis,” and “Type 1 diabetes.” They found no reported data associated with T1D and ocrelizumab when searching for “ocrelizumab” and “Type 1 diabetes.” Herein, the authors present a patient with MS who was diagnosed with T1D at the age of 8, whose HbA1c levels decreased with the initiation of ocrelizumab.
CASE REPORT
A 31-year-old female, diagnosed with T1D at the age of 8, was diagnosed with MS in October 2008 based on clinical presentations and results of further examinations, according to the 2005 McDonald criteria. Even though their only presenting symptom was diplopia, they experienced other symptoms, including right extremity dominant muscle weakness of limbs, unsteady gait, and difficulty speaking during the course their disease. The patient started treatment with interferon-β1a for 2 years and, due to the lack of efficacy, treatment was switched to fingolimod in 2011. As a result of persistent radiological activity and clinical progression, the patient’s treatment was changed to ocrelizumab in 2019 (Figure 1). They had an Expanded Disability Status Scale (EDSS) score of 4.5. They received standard dosing with two 300 mg infusions separated by 2 weeks, followed by 600 mg every 6 months thereafter.
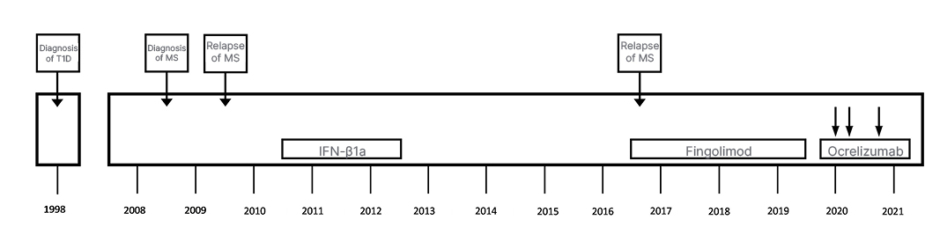
Figure 1: Treatment history of a 31-year-old female with Type 1 diabetes who was diagnosed with multiple sclerosis in 2008.
IFN-β1a: interferon-β1a; MS: multiple sclerosis; T1D: Type 1 diabetes.
The patient’s T1D course, on the other hand, had shown a fluctuating pattern throughout the 21 years since their primary diagnosis. Prior to the ocrelizumab therapy, their T1D was being treated with 62 IU/day of insulin (neutral protamine Hagedorn insulin and insulin glargine) on four injection regimens. The patient had also followed their diabetic dietary regime and individualised exercise programmes. Despite the patient’s strict treatment plan, their HbA1c levels were high in comparison to the target levels. Figure 2 illustrates a brief catch-up on the patient’s HbA1c levels and EDSS scores prior and after their ocrelizumab therapy. Within 3 months of initiating ocrelizumab therapy, a significant decrease was observed in the patient’s HbA1c levels. Besides their ocrelizumab therapy, no other potential factors (changes in T1D treatment protocols and lifestyle) could have been responsible for this decrease. Accordingly, a gradual 70% reduction was applied to their insulin doses. They are currently on 18 IU/day of a combination of insulin degludec/insulin aspart and insulin aspart on three injection regimens. The patient is currently stable on the course of both T1D and MS.
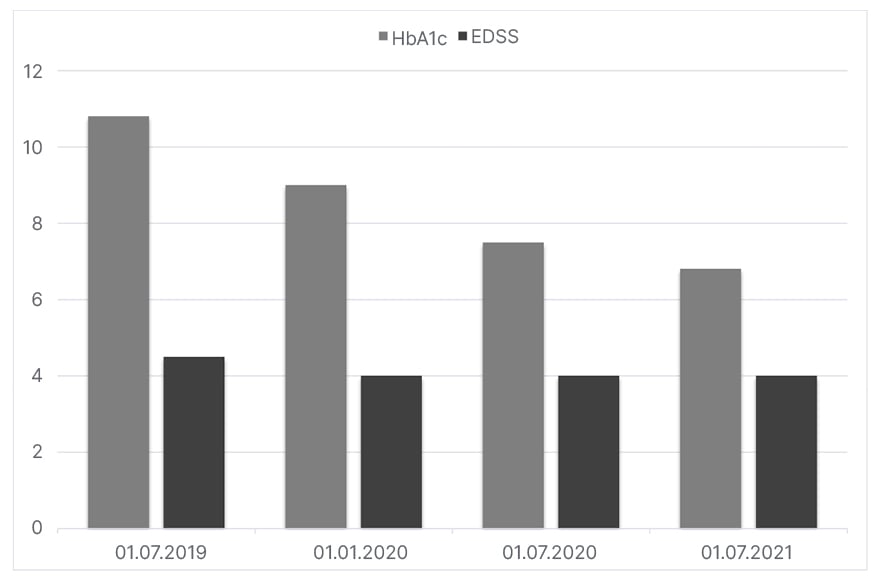
Figure 2: The HbA1c results of a 31-year-old female patient and their Expanded Disability State Scale score before and after treatment with ocrelizumab.
EDSS: Expanded Disability Status Scale.
DISCUSSION
The current data on the concurrence of MS and T1D has shown that the co-occurrence of the two diseases is not uncommon. Nielsen et al.4 demonstrated a relative risk of 3.26 (95% confidence interval: 1.80–5.88) for MS in thousands of patients with T1D in the Danish population. In this report, the authors presented a 31-year-old female patient with MS and a history of T1DM, whose initiation of ocrelizumab was sheerly due to their MS progression and independent of their T1D history. A noticeable improvement in the patient’s glycaemic control was observed within 3 months after ocrelizumab initiation. Ruling out other possible factors, the authors speculate that ocrelizumab could be responsible for the patient’s T1D course improvement.
Due to the important role of B cells in the pathogenesis of MS through antibody and cytokine production and antigen presentation, B cell depleting monoclonal antibodies against CD20 cell surface marker of B cells such as rituximab, ocrelizumab, and ofatumumab have revolutionised the treatment of MS.5 The mechanism of action of ocrelizumab, the first anti-CD20 monoclonal antibody approved for relapsing MS and first ever pharmacotherapy approved for PPMS, is thought to involve immunomodulation through a reduction in the number and function of CD20 expressing B cells.2
The use of disease modifying therapies in patients with MS has remarkably changed the management of the disease and has shown promising results for reducing the disease associated morbidity. Regarding the recent European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS)/ European Academy of Neurology (EAN) guidelines, ocrelizumab has shown a high quality of evidence for annual relapse rate reduction in patients with relapsing–remitting MS and for ≥24-week confirmed disability progression risk reduction in those with PPMS.6
The pathogenic destruction of insulin-secreting pancreatic β cells in T1D occurs via direct interaction with autoreactive T cells. The role of autoreactive T cells in T1D pathogenesis was defined long ago; therefore, autoreactive T cell targeting immunotherapy trials in T1D started to be conducted over 30 years ago.7 On the other hand, the role of B cells in T1D has been evidenced by different studies more recently, among which is the success of rituximab. It is a B cell targeting anti-CD20 monoclonal antibody that is used in patients with various CD20-expressing lymphoid malignancies, which delays T1D progression in both non-obese diabetic mice and patients with new-onset disease.8 In randomised controlled trials, several biological agents targeting T cells (cluster of differentiation 3 antibodies, lymphocyte function-associated antigen 3 antibodies, and anti-lymphocyte serum), B cells (rituximab), and T cell-associated co-stimulation pathways improved β cell functions in different magnitudes and durations.9 To the authors’ best knowledge, no study has yet been conducted to measure the effects of ocrelizumab on T1D.
In order to draw definite conclusions on this possible effect of ocrelizumab, clinicians managing patients with MS should be aware of the effects in glycaemic control while using this drug on patients with MS and T1D.
The authors report, to the best of their knowledge, the first case of a patient with concomitant MS and T1D whose glycaemic control was improved after the initiation of ocrelizumab for MS treatment. Further studies may be of interest to evaluate the mechanism and impact of ocrelizumab in glycaemic control.