Hypovitaminosis D is associated with an increased prevalence and incidence of metabolic syndrome and Type 2 diabetes mellitus (T2DM).1 Moreover, meta-analyses of observational studies have consistently found that vitamin D deficiency is associated with an increased risk of cardiovascular mortality and morbidity.2,3 Although vitamin D levels are influenced by modifiable determinants, such as diet and sun exposure, classical twin studies showed that vitamin D levels are heritable in 50–80% of cases,4 thus implying the central role of genetic determinants.
Genes modulating vitamin D metabolism are likely candidates for the control of vitamin D levels. A large meta-analysis of a genome-wide association study of serum 25-hydroxyvitamin D identified variants in three genes involved in vitamin D metabolism: DHCR7, CYP2R1, and GC.5 DHCR7 encodes the enzyme 7-dehydrocholesterol reductase, thereby affecting vitamin D synthesis;6 CYP2R1 encodes a hepatic microsomal enzyme responsible for vitamin D 25-hydroxylation;7 and GC encodes for a multifunctional serum glycoprotein that binds and transports vitamin D.8 All genetic associations between these three genes and vitamin D levels were only observed in the general population and never in T2DM populations.
Our aim was to investigate the role of DHCR7, CYP2R1, and GC gene variants on serum vitamin D concentrations in a large and very homogeneous cohort of Italian patients with T2DM. DHCR7, CYP2R1, and GC genes were studied in 2,163 consecutive study subjects from the Sapienza University Mortality and Morbidity Event Rate (SUMMER) study, which was conducted in a cohort of patients with diabetes.9
The three genes were significantly associated with lower vitamin D levels, with a mean reduction of 4 ng/mL in carriers of the risk genotypes compared to wild-type carriers (Table 1). The allelic risk (OR) for vitamin D insufficiency (i.e., having a vitamin D level of <30 ng/mL) was 1.28 (p=0.003) for DHCR7, 1.36 (p=0.00047) for GC, and 1.18 (p=0.042) for CYP2R1.
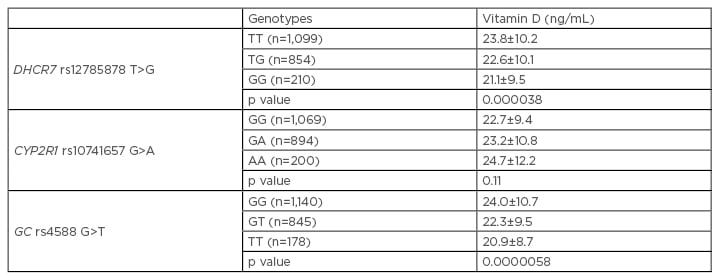
Table 1: Vitamin D levels of all participants in the SUMMER study across DHCR7, CYP2R1, and GC genotypes.
A: adenosine; C: cytosine; G: guanine; T: thymine.
Adapted from Barchetta et al.9
A weighted genotype risk score was then calculated by summing the risk alleles of the three single nucleotide polymorphisms in each individual, weighted with the effect size for risk of hypovitaminosis D (<30 ng/mL). We observed a strong association with vitamin D levels, which decreased significantly from the first to the last category (24.3±11, 24.8±11.5, 22±8.9, and 21±9 ng/mL, respectively [p=0.0000001]), with an OR for the subgroup with ≥3 risk alleles (versus 0 alleles carriers) of 1.24 (p=0.000011).
In summary, we observed a significant association between variants in the DHCR7, CYP2R1, and GC genes and lower vitamin D levels in T2DM patients. The weighted genotype risk score with the three variants together resulted in highly significant associations with vitamin D levels and with the risk of hypovitaminosis. Of note, we observed a mean difference between genotypes of 4 ng/dL in vitamin D levels. Although it is reasonable to believe that this difference between genotypes in vitamin D level may not be clinically relevant, it is conceivable that in diabetic subjects who are already affected by low vitamin D levels, a further genetically induced decrease may have detrimental consequences in the long term.
In conclusion, these results provide strong evidence of the effects of variations in genes coding for proteins involved in vitamin D metabolism on vitamin D levels in T2DM patients. Hence, we now have a genetic marker of vitamin D levels in diabetes that can be used in longitudinal studies to address whether the reported association between vitamin D and mortality rate10 in T2DM is sustained by a cause–effect relationship.








