Subcutaneous semaglutide once weekly (OW) and subcutaneous liraglutide once daily (OD) are two glucagon-like peptide-1 receptor agonists (GLP-1RA) approved for the treatment of Type 2 diabetes mellitus (T2DM).1,2 Both drugs decreased HbA1c, reduced body weight, and provided cardiovascular benefits in pivotal Phase III trials.3 Given the positive efficacy profiles and recent recommendations from international associations,4,5 both semaglutide and liraglutide are GLP-1RA that clinicians may consider prescribing to patients with T2DM; the clinical decision making involves choosing between an established OD formulation and a recently approved OW formulation that may be perceived as more convenient. Comparative head-to-head data would help resolve this clinical prescribing dilemma and provide evidence relevant to clinical practice. However, prior to the SUSTAIN 10 clinical trial, a Europe-based head-to-head comparison of the two drugs had not been conducted. SUSTAIN 10 compared the efficacy and safety of semaglutide 1.0 mg OW with that of liraglutide 1.2 mg OD. The doses were chosen to represent the most common (liraglutide 1.2 mg)6 and the anticipated most common (semaglutide 1.0 mg) prescription patterns in Europe and, thereby, increase the clinical relevance of the results.
This Phase IIIb, 30-week, open-label trial was conducted in 11 European countries and included 577 subjects with T2DM inadequately controlled with 1–3 antidiabetic drugs, i.e., metformin, sulphonylurea, and sodium–glucose cotransporter-2 inhibitors. Subjects were randomised 1:1 to subcutaneous semaglutide 1.0 mg OW or subcutaneous liraglutide 1.2 mg OD.
The primary and confirmatory secondary endpoints were, respectively, change from baseline to Week 30 in HbA1c and body weight. Other efficacy endpoints, together with safety and patient-reported outcomes, were also assessed.
The main results are shown in Table 1. Semaglutide was superior to liraglutide for the primary and confirmatory secondary endpoints: from baseline to Week 30, HbA1c decreased by 1.7%-point versus 1.0%-point (baseline 8.2%), and weight decreased by 5.8 kg versus 1.9 kg (baseline 96.9 kg), with semaglutide versus liraglutide, respectively (both p<0.0001). The proportions of subjects achieving HbA1c targets (<7.0% and ≤6.5%), weight-loss responses (≥5% and ≥10%), and clinically important composite endpoints were higher with semaglutide versus liraglutide (p<0.0001 for all). Similar results were seen with other glycaemic and weight parameters. Treatment satisfaction (as measured by the Diabetes Treatment Satisfaction Questionnaire [status version] summary score) improved with both treatments (estimated treatment difference 0.63; p=0.0814), and although statistical significance was not reached with all parameters, the data favoured semaglutide versus liraglutide.
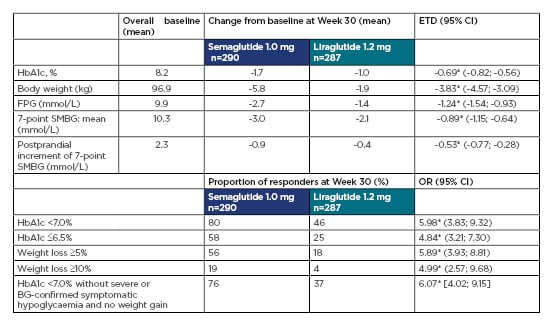
Table 1: Key primary and secondary endpoints in SUSTAIN 10.
*p<0.0001.
BG: blood glucose; CI: confidence interval; ETD: estimated treatment difference; FPG: fasting plasma glucose; HbA1C: haemoglobin A1C; OR: odds ratio; SMBG: self-measured blood glucose.
Safety profiles were generally similar with both treatments, except that higher proportions of subjects with semaglutide versus liraglutide experienced gastrointestinal adverse events (AE): nausea: 21.8% versus 15.7%; diarrhoea: 15.6% versus 12.2%; vomiting: 10.4% versus 8.0% and AE leading to treatment discontinuation (11.6% versus 6.6%; driven by gastrointestinal AE). These findings are consistent with the known safety profile of GLP-1RA: the most common AE observed with this class are gastrointestinal.7
In summary, SUSTAIN 10 confirmed that both treatments were effective in reducing HbA1c and body weight in subjects with T2D, and demonstrated superior efficacy with semaglutide 1.0 mg OW compared with liraglutide 1.2 mg OD. Both treatments were generally well-tolerated. These findings are consistent with those from the SUSTAIN 3 and 7 trials, which compared semaglutide with other GLP-1RA (exenatide extended release and dulaglutide, respectively).8,9 In conclusion, the SUSTAIN 10 results support semaglutide as a favourable treatment option in clinical practice.








