BACKGROUND AND AIMS
Type 1 diabetes (T1D), coeliac disease (CD), and autoimmune thyroid disease (AITD), abbreviated as TRIAD diseases, are three common autoimmune diseases with onset in childhood and adolescence.1 CD and AITD share high-risk genes with T1D, so a patient with T1D will have an increased risk of additionally being diagnosed with these diseases.2,3 TRIAD diseases are predictable in the prodromal phases with the potential to stratify the risk of disease by early autoimmune biomarkers. Autoantibodies (AAB) are key biomarkers for the identification of at-risk individuals prior to the appearance of clinical symptoms.4 The future goal is to establish a public screening programme identifying people who will develop a TRIAD disease later in life. Thus, this study aimed to test a recruitment model, home capillary blood sampling, and laboratory analyses.
MATERIALS AND METHODS
A total of 1,420 first-degree relatives (0–18 years, established biobank cohort of siblings) to newly diagnosed childhood/adolescent patients with T1D in Denmark, and 2,271 children (two age cohorts: 7–8 and 13–14 years) from the general population in Skåne were included in the study. Participants from the general population in Skåne received a home capillary kit for blood sampling with a video guide and returned the sample by public mail later. The antibody detection by agglutination-PCR (ADAP) multiplex technology as first-stage screening was compared with the gold standard radio binding assay (RBA) for the analysis of six AAB (glutamic acid decarboxylase, insulin, protein phosphatase-like IA-2 [IA-2A], zinc transporter 8 [ZnT8], thyroperoxidase, and tissue transglutaminase). Both Danish and Swedish samples were screened using the ADAP technology, and AAB positivity was confirmed by RBA.
RESULTS
From the Danish samples, 193 (13.6%) were positive for a TRIAD AAB, 107 (7.5%) were positive for a T1D AAB, 55 (3.9%) were positive for a CD AAB, 55 (3.9%) were positive for AITD AAB, and 65 (4.6%) were positive for ≥2 T1D AAB. In the Swedish study, 19,593 children were invited, and of those 2,271 individuals were included. From these, 211 (9.3%) were positive for a TRIAD AAB, 60 (2.6%) were positive for a T1D AAB, 61 (2.7%) were positive for a CD AAB, 99 (4.4%) were positive for AITD AAB, and 14 (0.6%) were positive for ≥2 T1D AAB. For 2,271 samples, ADAP and RBA were compared, and agreement ranged from 97.1– 99.3% (Figure 1).
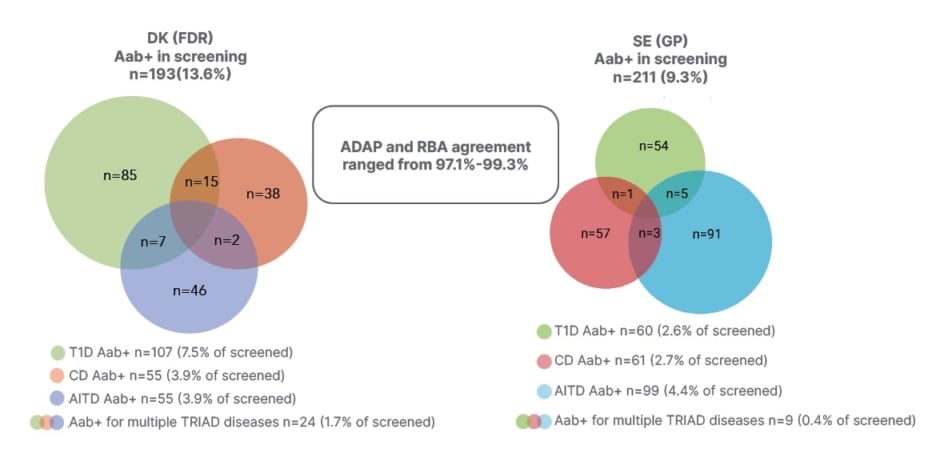
Figure 1: TRIAD autoantibody positivity in Danish first-degree relatives and Swedish general paediatric population.
Aab+: autoantibody positive; ADAP: antibody-detection by agglutination PCR; AITD: autoimmune thyroid disease; CD: coeliac disease; DK: Danish; GP: general paediatric population; FDR: first-degree relatives; RBA: radio binding assay; SE: Swedish; T1D: Type 1 diabetes.
CONCLUSION
The study confirms the high prevalence of associated autoimmunity in first-degree relatives, which supports the relevance of screening for multiple autoimmune diseases in this population. From these results, the authors can conclude that they have established an effective recruitment model with the participants being able to collect the screening sample at home. Thus, the authors’ model has the potential to be implemented as a large-scale screening programme identifying high-risk individuals. Moreover, it was shown that the ADAP method was comparable to the conventional RBA for the detection of AAB associated with TRIAD and can be applied as a first-stage screening method.







