ABSTRACT
Both glucagon-like peptide-1 receptor agonists (GLP-1RA) and sodium–glucose cotransporter-2 inhibitors (SGLT-2i) are effective and well-tolerated treatment options approved for the treatment of Type 2 diabetes mellitus (T2DM).1,2 The T2DM guidelines recommended them as second-line therapy after metformin use.3 Head-to-head trials between GLP-1RA and SGLT-2i are scarce, so little information is available to prescribers and patients to guide an informed choice between these two options. Subcutaneous once-weekly (OW) semaglutide, a GLP-1RA, has demonstrated superior glycaemic control and body weight reductions versus placebo and active comparators across the SUSTAIN clinical trial programme1,4,5 Canagliflozin, an SGLT-2i, also has proven efficacy for glycaemic control and weight loss compared with placebo and active comparators.6–8 Additionally, both OW semaglutide and canagliflozin have shown cardiovascular (CV) benefits in T2DM patients with high CV risk.9,10 The SUSTAIN 8 trial was undertaken as a head-to-head comparison of subcutaneous OW semaglutide with oral once-daily (OD) canagliflozin in patients with T2DM uncontrolled with metformin.
SUSTAIN 8 was a Phase IIIb, randomised, double-blind, double-dummy, active-comparator, parallel-group trial, conducted worldwide across 11 countries. The trial included 788 adults with T2DM and HbA1c 7–10.5%, randomised 1:1 to receive either semaglutide 1.0 mg OW and canagliflozin placebo OD, or canagliflozin 300 mg OD and semaglutide placebo OW for 52 weeks. Primary and confirmatory secondary endpoints included changes from baseline in HbA1c and body weight, respectively. Other efficacy endpoints, along with safety, were also assessed.
The key results from the trial are reported in Table 1. OW semaglutide was superior to canagliflozin for the primary endpoint of reduction in HbA1c (-1.5%-point versus -1.0%-point). Estimated treatment difference was-0.49%-point (95% confidence interval [CI]: -0.65; -0.33), p<0.0001. Semaglutide also led to superior reductions in body weight versus canagliflozin (-5.3 kg versus -4.2 kg). Estimated treatment difference was -1.06 kg (95% CI: -1.76;-0.36), p=0.0029. Greater proportions of subjects achieved HbA1c targets (<7.0% and ≤6.5%) and weight-loss responses (≥5% and ≥10%) with semaglutide versus canagliflozin (p<0.0001 for all [except the difference for weight-loss responses ≥5%; p=not significant]).
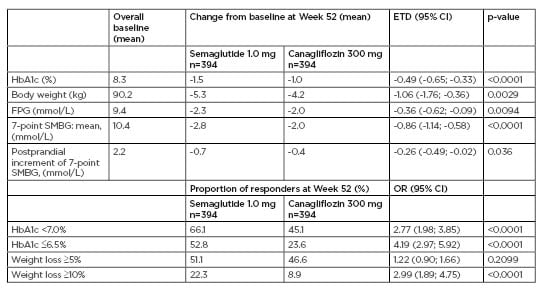
Table 1: Key primary and secondary endpoints in SUSTAIN 8.
CI: confidence interval; ETD: estimated treatment difference; FPG: fasting plasma glucose; HbA1c: haemoglobin A1c; OR: odds ratio; SMBG: self-measured blood glucose.
Overall, 76.0% of subjects treated with semaglutide and 71.8% of subjects treated with canagliflozin experienced adverse events. Consistent with previous SUSTAIN trials, the most frequent adverse events with semaglutide were gastrointestinal (46.9% versus 27.9% with canagliflozin), whereas the most frequent adverse events with canagliflozin were infections and infestations (34.5% versus 29.1% with semaglutide). The rates of premature treatment discontinuation due to adverse events were 9.7% (semaglutide) and 5.1% (canagliflozin), and severe or blood glucose-confirmed symptomatic hypoglycaemia occurred in 1.5% and 1.3% of subjects, respectively.
In summary, the SUSTAIN 8 trial provides clinically relevant information regarding the head-to-head comparison of a GLP-1RA and SGLT-2i in patients with T2DM. Semaglutide 1.0 mg OW demonstrated superior efficacy compared with canagliflozin 300 mg OD in patients treated with metformin who had uncontrolled T2DM. Both treatments were generally well tolerated, with low rates of hypoglycaemia. Gastrointestinal side effects were more common with semaglutide, while infections were more common with canagliflozin. These study outcomes may be used to guide clinical decision-making when treatment intensification is needed following metformin therapy in T2DM.








