Meeting Summary
Tildrakizumab is a monoclonal antibody with specificity for the p19 subunit of IL-23 that is approved for the treatment of moderate-to-severe plaque psoriasis in adults. Tildrakizumab was evaluated in two pivotal and parallel Phase III multicentre, double-blinded, randomised and placebo-controlled clinical trials: reSURFACE 1 and reSURFACE 2. These studies aimed to determine whether tildrakizumab is more effective than placebo and etanercept for the treatment of adult patients with moderate-to-severe chronic plaque psoriasis. Four post-hoc analyses from the reSURFACE 1 and reSURFACE 2 Phase III clinical trials were presented at the 28th European Academy of Dermatology and Venereology (EADV) Congress 2019. These post-hoc analyses investigated: 1) time to relapse in patients who had stopped treatment with tildrakizumab; 2) whether early responses to tildrakizumab could predict later responses; 3) whether disease duration affected response to tildrakizumab; and 4) long-term safety in patients >65 years of age. Median time to relapse after stopping tildrakizumab treatment was shown to be 32–37 weeks after the last dose. Early response to tildrakizumab (at Weeks 4, 8, and 16) showed a high predictability of continued success in later weeks (Week 28). Compared to etanercept, patients’ disease duration (≤5, >5 to ≤10, and >10 years) affected responses to tildrakizumab treatment less; however, patients receiving tildrakizumab who had a shorter disease duration at baseline did have higher efficacy rates at Week 28 than those who had longer disease durations before joining the studies. Lastly, up to Week 148, tildrakizumab was well-tolerated in patients >65 years of age.
INTRODUCTION
Psoriasis is a chronic, relapsing inflammatory skin disease, characterised in most cases by sharply demarcated, erythematous, pruritic plaques covered with silvery scales.1 Dysregulation of the innate and adaptive cutaneous immune responses is responsible for the development and maintenance of psoriatic inflammation in skin, and includes the IL-23/Th17 inflammatory pathway.1 The chronic nature of psoriasis necessitates long-term therapy, with mild-to-moderate psoriasis requiring topical treatment, and moderate-to-severe psoriasis usually requiring systemic treatment.2 Biologics targeting specific inflammatory pathways have been developed for the treatment of plaque psoriasis and currently focus on cytokines involved in the development of the disease, including IL-23, IL-17A, and TNF-α.1 IL-23, produced by dendritic cells, drives the expansion of Th17 cells and is a key cytokine in the development of psoriasis.1 The first biologic targeting IL-23 to be approved for psoriasis treatment was ustekinumab, which binds the p40 subunit of IL-23.1 However, as the p40 subunit of IL-23 is shared with IL-12, other immune mechanisms such as Th1 targeting are also affected by ustekinumab.1 Therefore, development of newer biologics targeting IL-23 alone have focussed mainly on the p19 subunit, as it does not bind IL-12.1 Three monoclonal antibodies with specificity for the p19 subunit of IL-23 have subsequently been developed and are either already approved or in clinical development: guselkumab, risankizumab, and tildrakizumab.3-5
Tildrakizumab, approved in 2018 for the treatment of adults with moderate-to-severe plaque psoriasis, was evaluated in two pivotal, parallel Phase III clinical trials: reSURFACE 1 and reSURFACE 2 and their long-term extension studies.6,7 In this article, the results of four post-hoc analyses of reSURFACE 1 and reSURFACE 2 are shared, which investigated: 1) time to relapse in patients who had stopped treatment with tildrakizumab; 2) whether early responses to tildrakizumab could predict later responses; 3) whether disease duration affected response to tildrakizumab; and 4) long-term safety in patients >65 years of age. These analyses were presented as posters at the EADV congress 2019.
The reSURFACE 1 and reSURFACE 2 Phase III Trials
The reSURFACE 1 and reSURFACE 2 studies
aimed to determine whether tildrakizumab is better than placebo and etanercept (a recombinant human fusion protein targeting TNF-α8) for the treatment of adult patients with moderate-to-severe chronic plaque psoriasis.6 In reSURFACE 1, 308 patients with moderate-to-severe psoriasis received 200 mg tildrakizumab, 309 received 100 mg tildrakizumab, and 155 received placebo (randomisation 2:2:1).6 In the parallel reSURFACE 2 study, 314 patients received 200 mg tildrakizumab, 307 received 100 mg tildrakizumab, 156 received placebo, and 313 received etanercept (randomisation 2:2:1:2).6 The studies were divided into three parts. Part 1 included Weeks 0–12, where patients were randomised as described above; Part 2 included Weeks 12–28, when patients who received placebo were rerandomised to receive 200 mg tildrakizumab or 100 mg tildrakizumab; and Part 3 included Weeks 28–64 for reSURFACE 1 and Weeks 28–52 for reSURFACE 2, when patients who responded to tildrakizumab were randomised (1:1) to either continue the same tildrakizumab dose or placebo (patients were retreated with the same tildrakizumab dose upon relapse).6 Tildrakizumab was administered subcutaneously to patients in Weeks 0, 4, and 16. Efficacy of tildrakizumab was analysed using the following primary endpoints: the proportion of patients achieving a Psoriasis Area and Severity Index (PASI) 75 (≥75% improvement in PASI) and Physician’s Global Assessment (PGA) response (score 0 or 1 with a reduction ≥2 degrees from baseline) at Week 12.6
The reSURFACE 1 and 2 trials included adults with moderate-to-severe plaque psoriasis affecting ≥10% body surface area, a PGA score of ≥3, and PASI ≥12. Baseline characteristics and demographics were similar across both studies and between the treatment arms.6 In the two trials, patients receiving tildrakizumab showed significantly higher efficacy results (PASI 75) compared with placebo (p<0.0001 for both 100 mg and 200 mg tildrakizumab versus placebo) and etanercept (p<0.0001 for 200 mg tildrakizumab versus etanercept; p=0.0010 for 100 mg tildrakizumab versus etanercept).6 At Week 12 of reSURFACE 1, 62% of the patients who received 200 mg tildrakizumab and 64% of patients who received 100 mg tildrakizumab achieved PASI 75, compared to 6% of patients in the placebo group.6 Regarding the PGA response, 59% of the patients who received 200 mg tildrakizumab and 58% of the patients who received 100 mg tildrakizumab achieved the desired PGA response, compared to 7% of patients in the placebo group. At Week 12 of reSURFACE 2, 66% of the patients who received 200 mg tildrakizumab and 61% of patients who received 100 mg tildrakizumab achieved the primary endpoint of PASI 75, compared to 6% of patients in the placebo group and 48% of patients in the etanercept group. Regarding the PGA response, 59% of the patients who received 200 mg tildrakizumab and 59% of the patients who received 100 mg tildrakizumab group achieved a PGA response, compared to 4% of patients in the placebo group and 48% of patients in the etanercept group.6 Tildrakizumab was well-tolerated by patients in both studies.6 The most common (≥1%) adverse effects included upper respiratory tract infections, injection site reactions, and diarrhoea.6
Post-Hoc Analysis of Time to Relapse in Patients Who Responded to Tildrakizumab Treatment in reSURFACE 1
Professor Kristen Reich
Long-term treatment is recommended for patients with psoriasis due to the chronic relapsing nature of the disease.2 Time to relapse, relapse rates after discontinuation of treatment, as well as predictors of relapse are therefore important features of novel biologics for treatment of psoriasis.
A post-hoc analysis aimed to evaluate time to relapse in patients who had received tildrakizumab in Parts 1 and 2 of reSURFACE 1, who were classified as responders to tildrakizumab at the end of Part 2 (Week 28), and who were then randomised to receive placebo for Part 3 (Weeks 28–64).9 The last dose of tildrakizumab these patients therefore received was at Week 16 and they were followed until the end of Part 3 of the study. In this post-hoc analysis, relapse was defined as loss of PASI 75 response between Weeks 28–64.9
At Week 28, 114 patients who were PASI 75 responders to 100 mg tildrakizumab and 119 patients who were PASI 75 responders to 200 mg tildrakizumab were randomised to receive placebo, with the option to be retreated with the same tildrakizumab dose upon relapse.6,9 From Week 28, the median time to relapse was 20 weeks with 100 mg tildrakizumab and 25 weeks with 200 mg tildrakizumab (32–37 weeks since the last 100–200 mg tildrakizumab dose).9 Median time to loss of PASI 90 (≥90% improvement in PASI) response was between 16–20 weeks (28–32 weeks since the last 100–200 mg tildrakizumab dose).9 A total of 20% of the patients who received 100 mg tildrakizumab and 24% of the patients who received 200 mg tildrakizumab did not relapse during the 36-week period (either they maintained PASI 75 or they were lost to follow up).9 No rebound of disease was observed (defined as worsening of psoriasis over baseline value [PASI>125%], or new pustular, erythrodermic, or more inflammatory psoriasis occurring within 2 months of stopping therapy).9
Characteristics of the patients who relapsed were also analysed, and smoking status, BMI, disease duration at baseline, and length of time sustaining PASI 90 were shown to be good predictors of relapse.9 Ex-smokers had 3.5-fold greater odds of relapse versus nonsmokers.9 The odds of relapse was 6% higher for every 1-unit increase in BMI, 3% higher for every 1-year increase in disease duration, and 1% lower for every week sustaining a PASI 90 response before Week 28.9
Pooled Post-Hoc Analysis from reSURFACE 1 and reSURFACE 2 Demonstrates That Tildrakizumab Can Provide an Early Predictability of Response
Professor Richard Warren
Response-guided therapy, particularly at early timepoints, is clinically useful for psoriasis treatment with biologics and can help guide better treatment decisions for patients. A pooled post-hoc analysis from reSURFACE 1 and reSURFACE 2 aimed to evaluate whether an early response to tildrakizumab at Weeks 4, 8, and 16 (PASI 50 response [≥50% improvement in PASI]) could predict a later response at Week 28, defined as either a PASI 90 response or an absolute PASI of <3.10
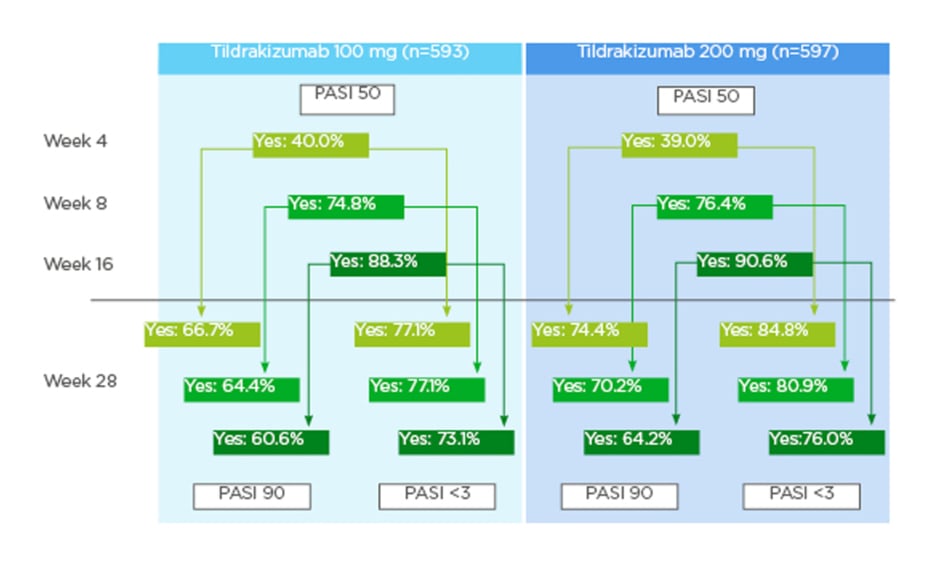
A total of 593 patients who received 100 mg tildrakizumab and 597 patients who received 200 mg tildrakizumab were analysed.10 PASI 50 was achieved by 40.0% and 39.0% of the patients who received 100 mg and 200 mg tildrakizumab, respectively, by Week 4; by 74.8% and 76.4% of patients who received 100 mg and 200 mg tildrakizumab, respectively, by Week 8; and, by 88.3% and 90.6% of patients who received 100 mg and 200 mg tildrakizumab, respectively, by Week 16.10
Response to tildrakizumab at earlier weeks showed a high predictability of continued success in later weeks.10 For those patients who achieved a PASI 50 at Week 4, 66.7% (100 mg tildrakizumab) and 74.4% (200 mg tildrakizumab) achieved a PASI 90 response and 77.1% (100 mg tildrakizumab) and 84.8% (200 mg tildrakizumab) achieved a PASI <3 at Week 28.10 For those patients who achieved a PASI 50 at Week 8, 64.4% (100 mg tildrakizumab) and 70.2% (200 mg tildrakizumab) achieved a PASI 90 response and 77.1% (100 mg tildrakizumab) and 80.9% (200 mg tildrakizumab) achieved a PASI <3 at Week 28.10 For those patients who achieved a PASI 50 at Week 16, 60.6% (100 mg tildrakizumab) and 64.2% (200 mg tildrakizumab) achieved a PASI 90 response and 73.1% (100 mg tildrakizumab) and 76.0% (200 mg tildrakizumab) achieved a PASI <3 at Week 28 (Figure 1).10 Additionally, lack of response to tildrakizumab by Weeks 8 and 16 was highly predictive of continued lack of response at Week 28.10 Patients who had not achieved PASI 50 response at Week 8 were unlikely to achieve PASI 90 response at Week 28; the corresponding rates were 21.8% (100 mg tildrakizumab) and 21.6% (200 mg tildrakizumab). Similarly, only 29.9% (100 mg tildrakizumab) and 33.6% (200 mg tildrakizumab) had PASI values <3 at Week 28.10 For patients who did not achieve PASI 50 response at Week 16, the corresponding response rates were 0.0% (regardless of dose) for PASI 90 at Week 28, and 14.5% (100 mg tildrakizumab) and 13.8% (200 mg tildrakizumab) for PASI <3 at Week 28.10
However, failure to achieve a PASI 50 by Week 4 did not necessarily predict a continued lack of response: of those patients who did not achieve a response at Week 4, 44.9% (100 mg tildrakizumab) and 47.9% (200 mg tildrakizumab) achieved a PASI 90 response at Week 28; the corresponding rates for PASI <3 were 57.1% (100 mg tildrakizumab) and 59.5% (200 mg tildrakizumab) (Figure 2).10
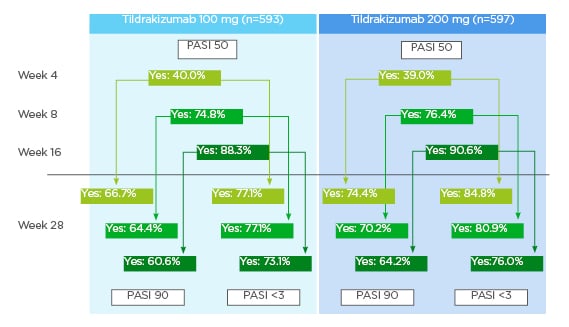
Figure 1: Patients with PASI 90 response or Psoriasis Area and Severity Index (PASI) <3 at Week 28 when PASI 50 was achieved at Weeks 4, 8, and 16.9
PASI: Psoriasis Area and Severity Index.
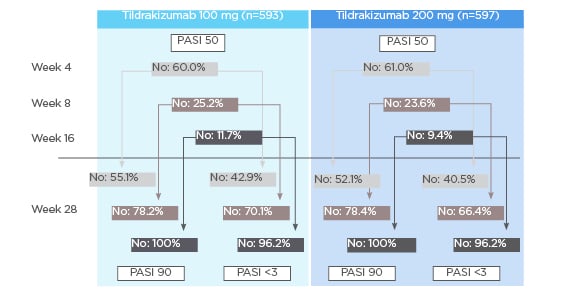
Figure 2: Patients without Psoriasis Area and Severity Index (PASI) 90 response or PASI <3 at Week 28 when PASI 50 was not achieved at Weeks 4, 8, and 16.9
PASI: Psoriasis Area and Severity Index.
Efficacy of Tildrakizumab According to Disease Duration at Baseline in a Pooled Post-Hoc analysis from reSURFACE 1 and reSURFACE 2
Professor Diamant Thaçi
The length of time patients have experienced psoriasis disease can affect their responses to certain biological treatments.11 A pooled post-hoc analysis from reSURFACE 1 and reSURFACE 2 therefore aimed to investigate whether disease duration (≤5, >5 to ≤10, and >10 years) at baseline affected patient responses to tildrakizumab.12 Response to tildrakizumab was determined by efficacy at Week 28: proportions of patients achieving PASI 75, PASI 90, and an absolute PASI <3.12 A total of 1,479 patients were included in this analysis: 273 patients with a disease duration ≤5 years, 266 patients with >5 to ≤10 years, and 940 patients with >10 years.12 Data from this post-hoc analysis are based on the full analysis set; missing data were handled using nonresponder imputation.12
Overall, compared to etanercept, patients receiving tildrakizumab demonstrated less difference between efficacy endpoints based on disease duration; however, patients treated with tildrakizumab who had experienced shorter disease duration did have higher efficacy rates than patients who had experienced longer disease durations.12 For patients receiving 100 mg tildrakizumab, at Week 28, PASI 75 was achieved in 81.2% (≤5 years disease duration), 76.4% (>5 to ≤10 years), and 72.6% (>10 years) of patients; PASI 90 was achieved in 61.5% (≤5 years), 53.8% (>5 to ≤10 years), and 48.2% (>10 years) of patients; and PASI <3 was achieved in 72.1% (≤5 years), 60.4% (>5 to ≤10 years), and 60.8% (>10 years) of patients.12
For patients receiving 200 mg tildrakizumab at Week 28, PASI 75 was achieved in 83.3% (≤5 years disease duration), 71.2% (>5 to ≤10 years), and 74.9% (>10 years) of patients; PASI 90 was achieved in 67.6% (≤5 years), 53.3% (>5 to ≤10 years), and 54.7% (>10 years) of patients; and PASI <3 was achieved in 74.1% (≤5 years), 65.4% (>5 to ≤10 years), and 66.2% (>10 years) of patients.12
For patients receiving etanercept at Week 28, PASI 75 was achieved in 60.5% (≤5 years disease duration), 45.3% (>5 to ≤10 years), and 54.4% (>10 years) of patients; PASI 90 was achieved in 46.5% (≤5 years), 24.5% (>5 to ≤10 years), and 26.9% (>10 years) of patients; and PASI <3 was achieved in 55.8% (≤5 years), 39.6% (>5 to ≤10 years), and 39.4% (>10 years) of patients.12
Pooled Post-Hoc Analysis from reSURFACE 1 and reSURFACE 2 Investigates Long-Term Safety of Tildrakizumab in Patients 65 Years of Age or Older
Professor Peter van de Kerkhof
Patients with psoriasis who are >65 years of age are more likely to suffer comorbidities and to develop adverse effects because of treatment than younger patients.13 A pooled post-hoc analysis from reSURFACE 1 and reSURFACE 2 therefore aimed to consider the long-term safety profile of tildrakizumab in patients who were >65 years of age.14 A total of 161 patients >65 years of age were exposed to tildrakizumab up to Week 148 (159.5 patient years [PY] of exposure to 100 mg tildrakizumab and 170.8 PY of exposure to 200 mg tildrakizumab).14 PY of exposure to etanercept was 14.7.14
Up to Week 148, tildrakizumab was well-tolerated in patients older than 65 years of age, with low drug-related serious adverse events and adverse events of special interest.14 In addition, no dose-related increase in the rate of adverse events was observed.14 The exposure adjusted incidence rates (EAIR; events per 100 PY of exposure) of drug-related serious adverse events were 2.51, 1.76, and 6.83 for patients receiving 100 mg tildrakizumab, 200 mg tildrakizumab, and etanercept, respectively.14 The EAIR of severe infections were 3.76, 2.34, and 6.83 for patients receiving 100 mg tildrakizumab, 200 mg tildrakizumab, and etanercept, respectively.14 The EAIR of malignancies (excluding nonmelanoma skin cancer) were 1.88, 1.76, and 6.83 for patients receiving 100 mg tildrakizumab, 200 mg tildrakizumab and etanercept, respectively.14 The EAIR of confirmed extended major adverse cardiovascular events (nonfatal myocardial infarction, non-fatal stroke, unstable angina, coronary revascularisation, resuscitated cardiac arrest, and cardiovascular deaths that were confirmed as “cardiovascular” or “sudden”) were 0.63, 1.17, and 6.83 for patients receiving 100 mg tildrakizumab, 200 mg tildrakizumab, and etanercept, respectively.14 Finally, the EAIR of injection site reactions were 0.63, 2.34, and 20.48 for patients receiving 100 mg tildrakizumab, 200 mg tildrakizumab, and etanercept, respectively.14
SUMMARY AND CONCLUSIONS
Tildrakizumab was approved in Europe and the USA in 2018 for the treatment of adults with moderate-to-severe plaque psoriasis.5,15 Two pivotal Phase III studies, reSURFACE 1 and reSURFACE 2, showed that tildrakizumab was efficacious and significantly more effective than placebo (p<0.0001 for both 100 mg and 200 mg tildrakizumab versus placebo) and etanercept (p<0.0001 for 200 mg tildrakizumab versus etanercept; p=0.0010 for 100 mg tildrakizumab versus etanercept), with a favourable safety profile.6 Four post-hoc analyses from reSURFACE 1 and reSURFACE 2 described here expand these results and provide more information on the clinical use of tildrakizumab.
Reich et al.9 showed that the median time to relapse after stopping tildrakizumab treatment was 32–37 weeks since the last dose, and that smoking status, BMI, disease duration, and length of time sustaining PASI 90 were good predictors of relapse. These data can help inform decisions about length of time patients continue to receive treatment and whether it is possible to stop treatment at any time.
Warren et al. demonstrated that early response to tildrakizumab (at Weeks 4, 8, and 16) showed a high predictability of continued success in later weeks (Week 28). Conversely, a lack of response by Weeks 8 and 16 predicted a continued lack of response; however, it is important to note that failure to achieve a response by Week 4 did not necessarily predict a continued lack of response. These data could be very useful in cost-effectiveness models and in clinical practice to help guide better treatment decisions for patients.10
Thaçi et al.12 showed that, compared to etanercept, disease duration at baseline affected responses to tildrakizumab treatment less; however, patients treated with tildrakizumab who had experienced shorter disease duration did have higher efficacy rates (PASI 75, PASI 90, and an absolute PASI <3 at Week 28) than those who had experienced longer disease durations. These data suggest that treating as early as possible might be beneficial for patients. However, further analysis could include other baseline characteristics such as failure on previous biologic use, as this could influence response rates and may be higher in patients with longer disease duration at baseline.
Lastly, van de Kerkhof et al.14 demonstrated that up to Week 148, tildrakizumab was well-tolerated in patients older than 65 years of age. As many patients with psoriasis are in this age group and may experience comorbidities, this data is helpful for informing treatment decisions for this age group.
In summary, these post-hoc analyses from the reSURFACE 1 and reSURFACE 2 studies provide key information that will help inform tildrakizumab treatment decisions for both clinicians and patients.