Meeting Summary
Prof Boehncke opened the symposium and highlighted the changing landscape of psoriasis management. On behalf of Prof Mrowietz, who was unable to attend, Prof Boehncke shared the evidence on fumarates in general and oral dimethyl fumarate (DMF) in particular. Dr Weisenseel closed the symposium by discussing the use of fumarates in current clinical practice.
Unmet Needs in Moderate-to-Severe Psoriasis
Professor Wolf-Henning Boehncke
The efficacy of biological agents compared with conventional systemic therapy in the management of moderate-to-severe psoriasis is well established.1 Recent evidence has confirmed the efficacy of newer biologicals, such as secukinumab versus ustekinumab, with a 75% improvement in Psoriasis Area Severity Index (PASI) 75 responses in up to 80% of patients at Week 16.2 However, the efficacy of these newer biologicals is tempered by the Candida infection safety signal, which was reported in the UNCOVER trials investigating ixekizumab.3
While efficacy and safety are important clinical outcomes, other factors also require consideration. A general observation is that even among patients who experienced complete clearance of their psoriasis symptoms, there is a significant subgroup (approximately 20%) who are not completely happy with their current situation, as reflected by a Dermatology Life Quality Index (DLQI) score >1. Further evidence from a survey of patients with well-controlled psoriasis indicates that the majority of patients continue to have unmet needs.4 In particular, patients on topical treatments and, consequently, the majority of patients would consider new therapies that might improve their overall quality of life.4
The German psoriasis guidelines issued in 2012 recognise the need for better therapeutic options for psoriasis, along with wider access to effective treatment.5 This was reiterated by a 2016 review, which highlighted that “…the potential for low satisfaction with psoriasis treatment, the rates of untreated and undertreated patients, frequent treatment switching, and the widespread use of combination therapy in the attempt to try to achieve a satisfactory response represent unmet treatment needs in patients with psoriasis of all severities.”6
Therefore, in addition to biological therapies, there is a need for well-tolerated, effective, cost-effective therapies for patients with mild-to-moderate psoriasis.7 Current innovations focus on this patient cohort, and fumarates, with their long-term efficacy, good safety profile, and established place in oral psoriasis therapy, have been identified as a possible management option for these patients.
Experience and New Data with the Use of Fumaric Acid Esters in the Treatment of Psoriasis
Professor Wolf-Henning Boehncke (on behalf of Professor Ulrich Mrowietz)
Fumaric acid esters (FAE) are licensed for the treatment of moderate-to-severe psoriasis in Germany. FAE have been available in Germany since the early 1990s and are the most frequently prescribed systemic agent for the treatment of plaque psoriasis.8 The results from two well-designed studies clearly demonstrate the efficacy of FAE relative to placebo, with a risk ratio that favours FAE: 4.55 (95% confidence interval: 2.80–7.40; p<0.00001).9 Head-to-head comparative data also confirm the efficacy of FAE in patients with psoriasis, with ≥50% of patients achieving a PASI 50 response rate from Week 12 to Week 24.10
Dimethyl Fumarate-only Formulation Recently Approved
A fixed combination of DMF plus three salts of ethyl hydrogen fumarate (Fumaderm®, Biogen Inc., Zug, Switzerland) is licensed in Germany for the treatment of psoriasis. DMF preparations contain ethyl hydrogen fumarate salts, which are not considered to substantially contribute to its efficacy,8 but a DMF-only formulation, known as Skilarence® (Almirall SA., Barcelona, Spain), was approved by the European Medicines Agency (EMA) in June 2017 for the treatment of moderate-to-severe plaque psoriasis in adults who require systemic therapy.11 The multicentre, double-blind, adaptive BRIDGE study randomised patients from 57 study sites in four European countries to two FAE drugs, Skilarence, Fumaderm, or placebo.12 Although the study protocol allowed up-titration from 30 mg/day to 720 mg/day, Dr Boehncke noted that in clinical practice there is greater flexibility in up-titration and down-titration. At baseline, all patients (mean age: 44–45 years) were categorised as moderate-to-severe, with a mean PASI score of 16, a mean body surface area of 21%, and with 42% of patients with a baseline DLQI of 11–20 (Almirall. 2017. Data on file).
At Week 16, the proportion of patients achieving a PASI 75 response with Skilarence was significantly higher than placebo (p<0.0001) and was non-inferior to Fumaderm (p=0.0003) (Figure 1). Significantly more patients treated with Skilarence also had a Physician’s Global Assessment score of 0 or 1 versus placebo (p<0.0001).12 Skilarence also improved quality of life with a mean improvement in the DLQI of 52% relative to baseline (Almirall. 2017. Data on file).12
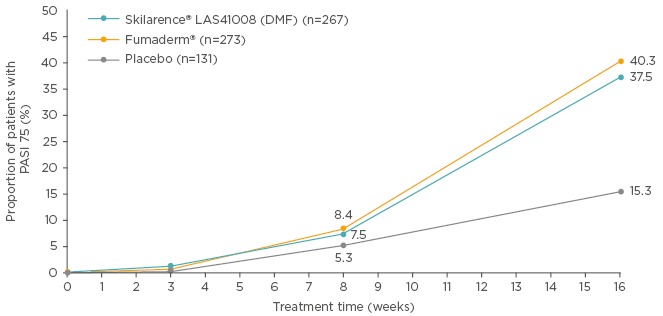
Figure 1: BRIDGE study: Proportion of patients with a Psoriasis Area Severity Index 75 response.
Data are presented for the full-analysis set, last observation carried forward.
DMF: dimethyl fumarate; PASI: Psoriasis Area Severity Index.
Adapted from Mrowietz et al.12
As expected, the adverse event (AE) profile of Skilarence was consistent with that of Fumaderm; treatment-related AE occurred more frequently in both treatments relative to placebo (Almirall. 2017. Data on file).12 The majority of AE, such as diarrhoea, abdominal pain, and flushing, occurred with a similar incidence for Skilarence and Fumaderm, were mild and did not lead to treatment discontinuation. Lymphocyte counts <700 cells/μL were detected in 5.4% and 6.4% of patients treated with Skilarence and Fumaderm, respectively, and lymphocyte counts <500 cells/μL were detected in 2.5% and 1.1% of patients treated with Skilarence and Fumaderm, respectively (Almirall. 2017. Data on file). Lymphocyte and leukocyte counts should be monitored on a 3-month basis forpatients treated with Skilarence; if a lymphocyte count below 1.0×109/L but ≥0.7×109/L is detected, monitoring should be conducted on a monthly basis until levels of ≥1.0×109/L are detected in two consecutive blood tests (after which point, the blood tests can be conducted every 3 months). Counts should be monitored on a monthly basis for those treated with Fumaderm.11
The place of fumarates in oral psoriasis therapy is well established. They are recommended for both the induction and long-term management of the disease in German and European guidelines based on 190,000 patient-years of experience.13 Skilarence is a DMF-only oral fumarate formulation that provides an efficacious and well-tolerated therapy for psoriasis. It has been approved in Europe as a first-line systemic treatment for moderate-to-severe plaque psoriasis.
Practical Clinical Tips on Managing Treatment with Adverse Effects
Doctor Peter Weisenseel
The majority of patients with psoriasis have mild disease; approximately one-third have moderate-to-severe disease.14 Therapeutic options for psoriasis depend on disease severity.14 Given the heterogeneity of patients with psoriasis, it is not possible to determine whether a patient’s treatment can be adjusted to achieve optimal efficacy and tolerability prior to initiating treatment. European consensus recommendations recommend assessing treatment efficacy at Week 16 for fast- acting systemics, such as infliximab or adalimumab, and at Week 24 for slower acting substances, such as methotrexate or fumarates.15 These consensus recommendations note the importance of waiting 24 weeks before assessing fumarate treatment efficacy.15 Despite 42% of psoriasis patients receiving at least three medications for non-psoriasis-related conditions,16 FAE do not have any direct drug–drug interactions due to their metabolism via unspecific esterases.17 Due to their potential influence on immune or kidney functions, certain drugs, such as retinoids, systemic psoralenes, cyclosporin, immunosuppressants, and cytostatics should not be combined with FAE.8
Flexible Dosing Schedule with Skilarence is Useful in Adverse Effect Management
The dosing scheme for FAE/DMF is initiated with a low-dose 30 mg DMF tablet for the first 3 weeks, ≤90 mg DMF/day. Thereafter, tablets containing 120 mg DMF are used for dosing ≤720 mg/day, although maximum daily doses of 360–480 mg/day are sufficient in many cases for the long-term management of the disease.8,11 After a stable clinical response has been achieved, a slow, stepwise down-titration to the minimum dose needed to maintain the clinical response is recommended in daily practice. Individualised dosing with 30 mg DMF tablets is also possible beyond Week 3. According to the Summary of Product Characteristics, Fumaderm and Skilarence are both associated with AE that can be clustered into four different categories: flushing, gastrointestinal (GI) AE, renal AE, and haematological AE.11 These potential AE require careful investigation and monitoring, and specific management strategies are available for each one.
Flushing is usually spontaneous and transient, occurs 1–5 hours after FAE/DMF administration, and typically lasts for 15–30 minutes.18 Flushing is caused by prostaglandin-mediated capillary vasodilatation and most commonly affects the face, but may also affect the neck and the upper chest.18 Patients should be advised that this may occur during the induction phase, but is not common during long-term therapy, and should not be attributed to an allergic reaction.18 To reduce the risk of flushing, patients are recommended to take the highest dose in the evening or, alternatively, it can be mitigated using oral prostaglandin inhibitors, such as acetylsalicylic acid (325–500 mg), taken as a single dose either before or after FAE.18 However, the combination of FAE and prostaglandin inhibitors is not recommended on a daily basis.18
GI adverse events are of importance since they can be bothersome for patients. GI AE are dose-dependent with FAE and usually occur when doses of DMF reach 120 or 240 mg/day.15 These AE are typically transient and decline with long-term therapy. A dose reduction to the last well-tolerated dose is recommended for patients who cannot tolerate GI AE. Once GI symptoms have become mild or very mild, an incremental up-titration may be resumed.15 The recommended dosing scheme involves taking the highest dose in the evening and with dairy (e.g., a glass of milk or yoghurt), to reduce the likelihood of diarrhoea.15 In some patients, antihistamines or proton-pump inhibitors may be beneficial, although this is not supported by clinical study data.
For example, the standard FAE/DMF dosing schedule for a patient with plaque psoriasis is to up-titrate from a starting dose of 30 mg/day to 120 mg/day after 4 weeks.11 According to Dr Weisenseel, individualised dosing in small increments is recommended to improve tolerability. Dosing should be adapted according to tolerability and clinical response.11,19 If the patient experiences flushing or GI AE, the schedule can be adjusted to deliver the main dose at noon or in the evening, depending on patient preference, when AE are less likely to affect activities of daily living; however, this approach is not recommended in the case of severe diarrhoea.
GI AE and flushing typically occur after 4 weeks of treatment. In a patient with mild adverse GI effects or flushing, a dose increase to 120 mg/day may induce moderate or even severe AE.18 For these patients, routine clinical management in Germany would involve an individualised approach: starting with a dose reduction until symptoms become mild, maintenance at the lower dose until adaptation to the AE occurs, and then slowly resume up-titration every second or third week to a higher dose, as tolerability allows. A second dose reduction may be required based on the severity of the AE. An alternative approach involves reducing the morning dose from 120 mg to 30 mg with incremental step-up treatment by 30 mg/week until the AE is considered to be mild.
Individualisation of Treatment is Possible with Fumaric Acid Ester/Dimethyl Fumarate
The flexible dosing schedule for FAE/DMF allows patients on long-term therapy to individualise their treatment in collaboration with their physician. Flexible dosing is particularly useful because disease activity in psoriasis often varies over time, with exacerbations often occurring in autumn or winter.
The retrospective FUTURE study20 (N=958) investigated flexible dosing in daily practice in patients with psoriasis who received FAE for ≥2 years. Within the first 3 weeks, 96.3% of patients were prescribed the recommended dosing schedule. In the dosing phase, 13.6% of physicians did not adhere to the recommended schedule, with only 34.9% of patients receiving the maximum dose of 720 mg/day (Figure 2).20 Therefore, the maximum dose is not required in most cases to achieve efficacy and the dose may be adjusted according to tolerability. In Germany, patients are typically treated with 360–480 mg/day.
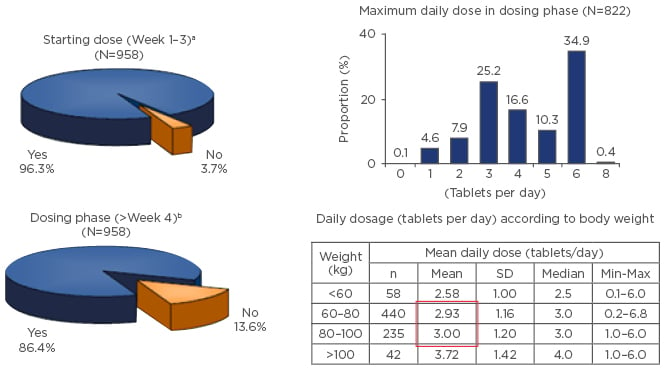
Figure 2: FUTURE study: Flexible dosing in daily practice.
aRecommended standard dosage scheme with increase of one tablet/week; bAn increase by one tablet/week.
SD: standard deviation.
Adapted from Reich et al.20
The FUTURE study highlighted that changes in laboratory values are uncommon and, when they do present, do not usually require action on the part of the physician (Figure 3).20
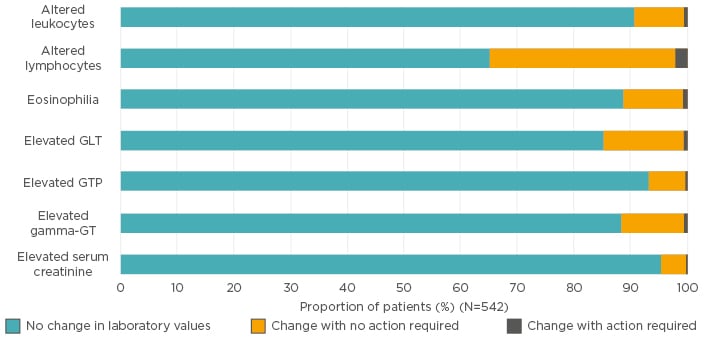
Figure 3: FUTURE study: Mean changes in laboratory values following long-term fumaric acid ester therapy over the entire data collection period (up to 36 months).
GLT: glutamate transporter; GT: glutamyltransferase; GTP: guanosine triphosphate.
Adapted from Reich et al.20
Fumaderm therapy requires laboratory monitoring (lymphocytes, leukocytes, urinalysis) at least every month, irrespective of clinical response or tolerability.8 Skilarence, however, only requires blood and urine assessment every 3 months depending on haematological values.11 For patients on Skilarence, monthly monitoring is necessary if lymphocyte counts fall below the normal range (700–1,000 cells/μL); therapy must be discontinued if the lymphocyte count is <700 cells/μL based on two tests.11 Treatment should not be initiated if leukocyte count is <3,000 cells/μL, and treatment should be discontinued if leukocyte counts fall to <3,000 cells/μL during treatment.
A recent single-centre, retrospective study involving 127 patients receiving FAE found that FAE are frequently associated with transient or persistent proteinuria, which is typically mild and not clinically significant.21 However, significant renal dysfunction is rare and usually reversible on dose reduction or discontinuation.
A retrospective analysis of the long-term PsoBest Registry data confirmed that FAE do not increase the risk of serious infections or malignancies.23 If monitoring requirements are followed, the risk of progressive multifocal leukoencephalopathy is generally thought to be very low. To date, 14 cases of progressive multifocal leukoencephalopathy during treatment with different kinds of FAE in psoriasis have been reported globally, with all cases except one presenting in patients with pronounced or severe lymphopenia (<700 cells/μL). The remaining case had been treated for 19 months without any lymphocyte count monitoring and the only lymphocyte count available was <800 cells/μL.23 The average and median duration of lymphocytopenia to progressive multifocal leukoencephalopathy symptom onset were, respectively, 31.2 and 26 months (range: 2–72 months). To avoid severe, persistent lymphopenia, regular monitoring should be carried out.8
To assess whether the mean and maximum daily dose of FAE could be reduced, a prospective study was carried out that combined FAE and ultraviolet light during the up-titration phase.24 An indirect comparison of the FAST24 and FUTURE20 studies suggested that FAE plus ultraviolet light therapy in the up-titration period was well tolerated and resulted in a lower maintenance dose (2.6 versus 2.9 tablets of 120 mg DMF/day, respectively) and a faster onset of action (PASI 75) with combination therapy.
Dr Weisenseel noted that careful selection of the ‘right patient’ for FAE therapy involves choosing patients with chronic, stable psoriasis who require first-line systemic therapy and who have exhibited good compliance with previous treatment. Ideally, the patient will have realistic treatment expectations with a view to obtaining good long-term psoriasis control rather than a fast onset of action.
FAE are established for long-term control of moderate-to-severe plaque psoriasis in Germany and recommended in the S3 European guidelines.5 Adherence depends on choosing the right patient and detailed information prior to starting treatment. Potential AE can usually be managed using individualised dosing and an initial combination with ultraviolet therapy results in faster clinical response and a lower FAE dose.
Question and Answer Session
When do lymphocytes return to normal after stopping dimethyl fumarate, and do lymphocytes return to normal in all patients?
Usually within approximately 3 months, although for some patients lymphocytes may normalise within 4 weeks. In select patients, it may take longer than 3 months, and these patients require monitoring. Prof Boehncke commented that in 15 years of prescribing Fumaderm, he has never had a patient that had not returned to normal, although in a minority of patients this may take over a year.
If a patient achieves PASI 75 on fumarate therapy and is satisfied on their current dose of 120 mg twice daily, do you increase the dose?
Prof Boehncke’s approach involves joint decision- making between the clinician and the patient. If the patient is satisfied, there is no need to increase the dose; however, ultimately it is the patient’s decision and sometimes patients are satisfied with less-than-perfect clear skin.
Following the approval of Skilarence, what will happen in Germany? Can you switch from fumarates to Skilarence?
Prof Weisenseel acknowledged that you can switch to Skilarence. He would tend to use the DMF-only formulation because there are certain differences, such as monitoring and the number of active ingredients, between the two formulations.







