Meeting Summary
Hidradenitis suppurativa (HS) is a chronic, recurrent follicular skin disease characterised by deep and painful dermal inflammatory nodules, abscesses, and draining tunnels. HS is one of the dermatological diseases with the greatest unmet medical need. Post hoc analyses from two identically designed Phase III trials of secukinumab in patients with moderate-to-severe HS (SUNSHINE and SUNRISE) were featured in multiple poster and oral presentations at the 32nd annual European Academy of Dermatology and Venereology (EADV) Congress in Berlin, Germany.
The SUNSHINE and SUNRISE studies represent the largest Phase III trials conducted in HS to date. In these studies, secukinumab demonstrated sustained efficacy with a favourable safety profile, with both trials meeting the primary endpoint. The analyses presented at the EADV Congress were conducted to assess concordance between efficacy endpoints (55% reduction in Hidradenitis Suppurativa Severity Scoring System [IHS4-55] response and Hidradenitis Suppurativa Clinical Response [HiSCR]); the impact of secukinumab on HiSCR 75, HiSCR 90, and HiSCR 100 endpoints; and the effect of secukinumab on draining tunnels and HS-related pain, as well as work productivity and activity impairment (WPAI). Strong concordance between IHS4-55 and HiSCR was found, and IHS4-55 was considered to be a suitable efficacy outcome for HS.
Secukinumab also provided clinically meaningful improvements compared with placebo, as determined by HiSCR 75, HiSCR 90, and HiSCR 100 endpoints by Week 16. These effects were sustained through Week 52. Furthermore, at Week 16, >80% of patients treated with secukinumab experienced no increase in the number of draining tunnels from baseline. Secukinumab also improved HS-related skin pain, and reduced the use of pain medication compared with placebo. Finally, treatment with secukinumab had a beneficial and sustained effect on presenteeism, absenteeism, and general work impairment due to HS. These data, taken together, indicate that secukinumab provides sustained disease control, sustained reduction in pain, and sustained improvement in WPAI and occupational performance up to Week 52.
Concordance Between IHS4-55 Response and HiSCR: A Post Hoc Analysis of the SUNSHINE and SUNRISE Phase III Randomised Trials of Secukinumab in Patients with Moderate-to-Severe Hidradenitis Suppurativa
Due to the clinical heterogenicity of HS, assessment of disease severity is complex, and over 20 different outcome measures have been developed.1,2 HiSCR is a well-recognised dichotomous score currently used in HS clinical trials to assess treatment effects, and assesses morbidity improvement, or lack of, between two time points.1-3 Response is classified as at least a 50% reduction from baseline in the abscesses and inflammatory nodules count, with no increase in abscesses or draining fistulae count.4,5 This endpoint cannot be applied to patients who have fewer than three inflammatory lesions, or >20 draining fistulae.2 HiSCR 75, 90, and 100 represent a ≥75%, ≥90%, and 100% decrease in abscess and inflammatory nodule count, respectively, versus baseline.
A further tool used as a secondary outcome measure in HS clinical trials is the IHS4, which provides a continuous severity score by assigning different weights to different lesion types.1,6 IHS4 disease severity bands classify HS into mild, moderate, or severe disease.1 IHS4 also allows quantification of draining tunnels in a validated manner.1,6 However, the need for dichotomous outcomes in clinical trials led to the development and validation of IHS4-55, a binary version of IHS4, based on a 55% decrease in the total score between two time points.7 Although IHS4-55 has been validated, the concordance between dichotomous outcomes and patient-reported outcomes has not yet been evaluated.7
Secukinumab is a monoclonal antibody that selectively neutralises IL-17A, and has been evaluated in the identically designed SUNSHINE and SUNRISE pivotal Phase III trials for the treatment of patients with moderate-to-severe HS.4 Both studies were randomised, double-blind, multicentre, clinical trials that assessed the efficacy and tolerability of secukinumab. Patients were randomised 1:1:1 to one of two subcutaneous secukinumab dosing regimens (300 mg every 2 weeks [SECQ2W] or every 4 weeks [SECQ4W]) for 52 weeks, or to placebo for 16 weeks. At Week 16, patients enrolled into the placebo group were subsequently randomised 1:1 to either SECQ2W or SECQ4W.4 Key inclusion criteria included at least five inflammatory lesions affecting at least two distinct anatomical areas at baseline, and diagnosis of HS ≥1 year prior to enrolment.4 Patients in the antibiotic stratum were permitted to enter the study on stable treatment with selected antibiotics. Individuals with a total fistulae count ≥20 at baseline, active inflammatory disease, or previous exposure to secukinumab or other IL-17(A)-biologics were excluded.4
Secukinumab has previously demonstrated sustained efficacy with a favourable safety profile in patients with moderate-to-severe HS in the SUNSHINE and SUNRISE trials, with both studies meeting the primary endpoint.4 A post hoc analysis was conducted to determine if IHS4-55 response was concordant with HiSCR and Dermatology Life Quality Index (DLQI) response. A DLQI response was defined as a decrease of ≥5 points from baseline. Concordance was defined as the proportion of patients whose responses for the two assessed parameters were consistent during a specific visit. Concordance at Week 16 and 52 was assessed between the following parameters: IHS4-55 and HiSCR response; IHS4-55 and DLQI response; and HiSCR and DLQI response.
A total of 1,084 patients were included in the assessment of concordance between IHS4-55 and HiSCR, and 905 patients were included in the IHS4-55 and DLQI, and HiSCR and DLQI concordance assessments.
Strong concordance between IHS4-55 and HiSCR response at Week 16 and Week 52 (≥85.7% in pooled analysis and by trial) was observed in all treatment arms. Moderate concordance between IHS4-55 and DLQI responses (57.1% to 66.2%), and between HiSCR and DLQI responses (54.6% to 71.4%) in all treatment arms was reported. These data indicate that IHS4-55 is a suitable efficacy outcome for HS, either on its own or in addition to HiSCR.
Effects of Secukinumab on HiSCR 75, HiSCR 90, and HiSCR 100 Endpoints in Patients with Moderate-to-Severe Hidradenitis Suppurativa: A Post Hoc Analysis of the SUNSHINE and SUNRISE Phase III Trials
While HiSCR is a commonly used endpoint, it has previously demonstrated high placebo response rates.8,9 In order to minimise these rates, higher threshold efficacy endpoints have been proposed.
A post hoc analysis was performed to assess the treatment effects of secukinumab on HiSCR 75, HiSCR 90, and HiSCR 100 endpoints in 1,084 patients enrolled in the SUNSHINE and SUNRISE trials (as described above).4 Results from Week 0–16 were based on multiple imputation data, and results from Week 16–52 were based on observed data. The sustainability of response was evaluated by investigating the proportion of patients who achieved HiSCR 75, HiSCR 90, and HiSCR 100 up to Week 52.
A numerically greater proportion of patients treated with secukinumab achieved HiSCR 75, HiSCR 90, and HiSCR 100 versus placebo at Week 16 in both trials. Response rates for the higher threshold efficacy endpoints seen at Week 16 were sustained, with a trend for improvement to Week 52, in both secukinumab treatment groups.
The majority of patients who achieved an HiSCR 75 at Week 16 maintained this response at Week 52. In SUNSHINE, 62.9% of patients receiving SECQ2W and 70.0% of patients in the SECQ4W group achieved HiSCR 75 at both Weeks 16 and 52. In SUNRISE, 71.4% and 64.4% of patients in the SECQ2W and SECQ4W achieved these endpoints, respectively. Furthermore, patients in the secukinumab group had higher HiSCR 90 and HiSCR 100 response rates versus placebo at Week 16, which were sustained to Week 52.
Treatment continuation was associated with improvement in HiSCR over time, as the majority of patients in the SECQ2W and SECQ4W arms who achieved HiSCR at Week 16 showed improvement to HiSCR 75 at Week 52. In SUNSHINE, 62.1% of patients receiving SECQ2W and 65.4% of patients in the SECQ4W group achieved a HiSCR 75 at Week 52 after achieving an HiSCR at Week 16. For patients participating in SUNRISE, 62.3% and 58.5% of patients receiving SECQ2W and SECQ4W achieved an HiSCR 75 at Week 52 after HiSCR at Week 16, respectively. Additionally, 35% and >25% of patients in the SECQ2W and SECQ4W arms who achieved HiSCR 75 at Week 16 showed improvements to HiSCR 90 and HiSCR 100 at Week 52 (Table 1).
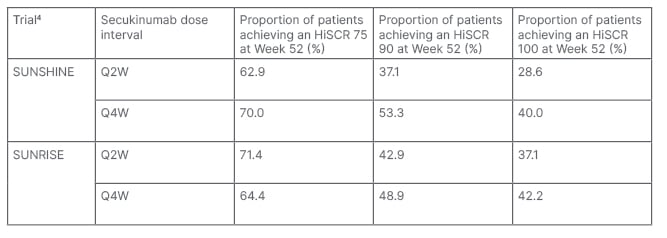
Table 1: Hidradenitis Suppurativa Clinical Response 75, 90, and 100 responses at Week 52 in patients receiving secukinumab who achieved Hidradenitis Suppurativa Clinical Response 75 at Week 16 (post hoc analysis).
HiSCR: Hidradenitis Suppurativa Clinical Response; Q2W: every 2 weeks; Q4W: every 4 weeks.
This investigation found that secukinumab provided clinically meaningful improvements compared with placebo in high threshold efficacy endpoints in both the SUNSHINE and SUNRISE trials,4 which were sustained through to Week 52. Long-term durability was demonstrated by the fact that >60% of patients receiving secukinumab who achieved HiSCR 75 at Week 16 maintained HiSCR 75 at Week 52 (Table 1), while approximately 60% of patients treated with secukinumab who achieved HiSCR at Week 16 further improved to HiSCR 75 at Week 52. These sustained improvements in HiSCR highlight the long-term benefits of secukinumab in patients with moderate-to-severe HS.
Effect Of Secukinumab on Draining Tunnels in Patients with Moderate-to-Severe Hidradenitis Suppurativa: Post Hoc Analysis of the SUNSHINE And SUNRISE Phase III Randomised Trials
The clinical presentation of HS can vary, with a wide range of inflammatory and non‐inflammatory lesions, including nodules, abscesses, draining tunnels, open pseudocomedones, scars, and ulceration.10 Draining tunnels in patients with HS cause considerable pain, and have a large negative impact on quality of life. Furthermore, they are associated with greater disease severity, irreversible tissue damage, and are predictors of poor response to therapy.11-14
A post hoc analysis of pooled data from the SUNSHINE and SUNRISE trials was conducted to assess the effect of up to 52 weeks of secukinumab treatment on draining tunnels.4 The exploratory endpoints assessed were mean change from baseline to Week 52 in the number of draining tunnels in patients with at least one draining tunnel at baseline, and the proportion of patients reporting no increase in draining tunnels from baseline to Week 52, in all patients and those with at least one draining tunnel at baseline.
All analyses were performed on pooled data from 1,084 patients in both trials, and are reported as observed. The mean±standard deviation (SD) number of draining tunnels at baseline was similar between treatment groups (SECQ2W: 2.90±3.51; SECQ4W: 2.50±3.51; placebo: 2.50±3.19). The majority of enrolled patients had at least one draining tunnel at baseline (Q2W: 66.2%; Q4W: 60.6%; placebo: 62.5%).
A numerically greater mean decrease from baseline in draining tunnels was reported in patients receiving secukinumab versus placebo at Week 16 (Figure 1), with the decrease sustained through to Week 52.
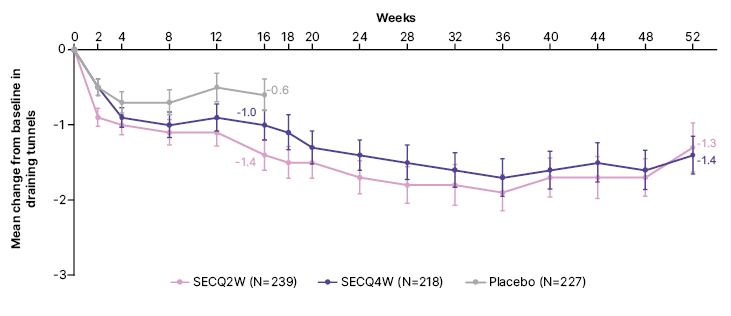
Figure 1: Mean change in draining tunnels over time up to Week 52 in patients with ≥1 draining tunnel at baseline (post hoc analysis).
Figure adapted from Bechara et al. EADV 2023.
Data are presented as observed. At Week 16, patients randomised to placebo were switched to receive SECQ2W or SECQ4W up to Week 52. Only patients on continuous secukinumab treatment for 52 weeks are represented in the graph beyond Week 16.
SECQ2W: secukinumab 300 mg every 2 weeks; SECQ4W: secukinumab 300 mg every 4 weeks.
Furthermore, a numerically greater proportion of patients treated with secukinumab versus placebo experienced no increase in the number of draining tunnels from baseline to Week 16 (SECQ2W: 84.5%; SECQ4W: 80.9%; placebo: 75.5%). This effect was sustained through to Week 52, with 80.7% of SECQ2W and 80.9% of SECQ4W patients experiencing no increase in draining tunnels between Week 16 and Week 52 (data not reported for placebo).
When assessing patients with at least one draining tunnel at baseline, a greater proportion of patients in the secukinumab groups versus placebo experienced no increase in the number of draining tunnels at Week 16 (SECQ2W: 82.9%; SECQ4W: 78.2%; placebo: 71.2%). This effect was sustained until Week 52 (SECQ2W: 80.7%; SECQ4W: 82.6%; placebo data not reported).
This analysis demonstrates the sustained effectiveness of secukinumab in reducing the number of draining tunnels in patients with moderate-to-severe HS. Moreover, >80% of patients treated with secukinumab experienced no increase in the number of draining tunnels from baseline to Week 52. These findings are of clinical relevance, as skin tunnel formation is associated with HS disease progression, and irreversible tissue damage.13,14
Secukinumab Provides Sustained Improvements in Pain in Patients with Moderate-to-Severe Hidradenitis Suppurativa: A Post Hoc Analysis of the SUNSHINE and SUNRISE Phase III Trials
Disease-related pain is one of the most detrimental symptoms for patients with HS.16 The effects of secukinumab on worst HS-related pain in patients with moderate-to-severe HS, using data from the SUNSHINE and SUNRISE trials, was evaluated in a post hoc analysis.4 Worst skin pain in the 24 hours prior to the visit (daily up to Week 16, and weekly thereafter) was assessed via the patient’s global assessment of skin pain on a continuous Numeric Rating Scale (NRS; 0–10 scale). Categories of worst HS-related skin pain were as follows: NRS=0 (no pain); NRS >0–≤6; NRS >6–≤8; and NRS >8 (severe pain). A mixed-effects model for repeated measures was used to assess the change in NRS scores from baseline to Week 16. The proportion of patients who reported the use of pain medication for HS was also evaluated at 28-day intervals up to Week 16. The achievement of a DLQI response by pain categories at Week 16 was the predefined exploratory endpoint.
Overall, 1,084 patients from the SUNSHINE and SUNRISE trials were included in this analysis.4 The mean NRS±SD pain score at baseline was 5.3±2.5, 5.1±2.5, and 5.2±2.5 in the SECQ2W, SECQ4W, and placebo groups, respectively.
This study found a numerically greater mean reduction from baseline in pain among patients treated with both secukinumab doses compared with the placebo group. At Week 16, the greatest reduction in NRS was seen among SECQ2W patients compared with SECQ4W or placebo (SECQ2W: -1.4±2.2; SECQ4W: -1.1±2.0; placebo: -0.5±2.1). Improvements were sustained through to Week 52, with a change from baseline in NRS of -1.8±2.6 for SECQ2W and -1.5±2.7 for SECQ4W. Patients who switched from placebo to secukinumab also experienced a reduction in skin pain from Week 16 to Week 52 (change in NRS: placebo to SECQ2W: -1.7±2.8; placebo to SECQ4W: -1.6±2.5).
The adjusted mean change from baseline in NRS at Week 16 was -1.3, -1.1, and -0.5 in patients receiving SECQ2W, SECQ4W, and placebo, respectively. The estimated treatment differences between secukinumab and placebo in the change from baseline in NRS score at Week 16 were -0.74 (95% confidence interval: -1.06, -0.42) for SECQ2W and -0.56 (95% confidence interval: -0.88, -0.23) for SECQ4W.
This analysis also evaluated the improvement in pain severity between baseline and Week 52. A small proportion of patients who received SECQ2W with an NRS of >6–≤8 and an NRS of >8 at baseline reported an NRS=0 (baseline NRS: >6–≤8, 8.3%; NRS: >8, 8.6%). Additionally, 63.3% of patients with NRS of >6–≤8, and 45.7% of patients with NRS of >8 at baseline, reported an NRS of >0–≤6 at Week 52. For those patients in the SECQ4W group with an NRS of >6–≤8 or NRS of >8 at baseline, 13.6% and 0.0% had an NRS=0, and 67.8% and 46.4% had an NRS of >0–≤6, respectively.
Use of any pain medication was reduced from 42.7% at baseline to 25.7% at Week 16 for SECQ2W patients, and from 36.7% to 19.9% for SECQ4W patients (Figure 2). For patients in the placebo group, the reported use of pain medication was lower at Week 16 (28.9%) compared with baseline (38.6%). A greater proportion of DLQI responders was observed in the lower pain categories at Week 16 (NRS=0: 70.6%; NRS: >0–≤6, 44.2%; NRS: >6–≤8, 28.1%; NRS: >8, 13.5%).
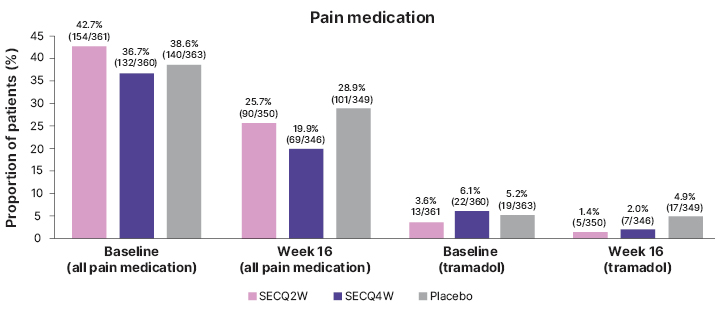
Figure 2: Frequency of pain medication use in patients enrolled in the SUNSHINE and SUNRISE trials (pooled) from baseline to Week 16 (post hoc analysis).
Figure adapted from Ingram et al. EADV 2023.
Percentage (n/N) are presented, where N represented the number of patients in the group, and n represents the number of patients who used pain medication for HS.
HS: hidradenitis suppurativa; SECQ2W: secukinumab 300 mg every 2 weeks; SECQ4W: secukinumab 300 mg every 4 weeks.
This analysis showed that secukinumab improved HS-related skin pain at Week 16 compared with placebo, with a trend for further improvement through to Week 52. These data demonstrate that patients receiving secukinumab experienced a reduction both in pain and in use of pain medication over time, with improved quality of life.
Impact of Secukinumab on Hidradenitis Suppurativa-Related Work Productivity and Activity Impairment: A Post Hoc Analysis of the SUNSHINE and SUNRISE Phase III Trials
HS exerts a substantial negative impact on patient’s quality of life, and is associated with a considerable decrease in an individual’s work ability and productivity, both of which result in a large socioeconomic burden.10,18-22 The impact of secukinumab on WPAI in patients with HS is unknown. Work productivity loss, presenteeism, and absenteeism in employed individuals, and activity impairment in all patients, was evaluated using the WPAI specific-health problem questionnaire at six time points (baseline, and Weeks 2, 16, 28, 44, and 52) in a post hoc analysis of the SUNSHINE and SUNRISE Phase III trials.4 The proportion of patients achieving a ≥20% improvement in work productivity loss and activity impairment was considered to represent a minimal clinically important difference.22 HiSCR and DLQI response (≥5-point improvement in total score compared with baseline) was measured as a correlative analysis with WPAI parameters.
This investigation included 972 patients who completed the WPAI-specific-health problem questionnaire at baseline. The majority of patients were employed (64.6%) and had Hurley Stage II (59.6%) or Stage III (36.6%), with a mean±SD abscess and inflammatory nodule count of 13.1±8.9, mean±SD time since HS symptom onset of 13.0±9.6 years, and a mean±SD time since HS diagnosis of 7.2±7.3 years. Patients reported a substantial mean work productivity loss of 40.2%, and a substantial mean activity impairment of 45.0% at baseline.
At Week 16, compared with placebo, patients in both secukinumab treatment groups had a mean absolute change from baseline improvement in work productivity loss (SECQ2W: -11.7%; SECQ4W: -8.7%; placebo: -1.0%), activity impairment (SECQ2W: -14.0%; SECQ4W: -10.0%; placebo: -3.6%), presenteeism (SECQ2W: -11.9%; SECQ4W: -9.1%; placebo: -1.6%), and absenteeism (SECQ2W: -5.9%; SECQ4W: -1.1%; placebo: 0.1%).
In general, responses with secukinumab treatment were sustained through to Week 52, with a trend for improvement over time. Additionally, patients who switched from placebo to secukinumab at Week 16 commonly exhibited an improvement in all WPAI outcomes through to Week 52.
A greater proportion of patients in either secukinumab group achieved a ≥20% improvement in work productivity loss (SECQ2W: 62.7%; SECQ4W: 48.0%; placebo: 41.8%) and activity impairment (SECQ2W: 62.9%; SECQ4W: 54.1%; placebo: 46.7%) versus placebo at Week 16. Patients receiving secukinumab improved the response at Week 52 for work productivity loss (SECQ2W: 71.1%; SECQ4W: 60.3%) and activity impairment (SECQ2W: 71.1%; SECQ4W: 63.7%), including patients who switched from placebo to secukinumab.
Patients treated with secukinumab who achieved an HiSCR and DLQI response at Week 16 and Week 52 had numerically higher WPAI improvements in all parameters, compared with patients not achieving these endpoints. However, improvements were still observed in patients not achieving HiSCR or a DLQI response, indicating that secukinumab may have an impact on work productivity in the context of minimal other clinical responses.
The authors concluded that in patients with moderate-to-severe HS, secukinumab has a beneficial effect on WPAI, with the improvement being sustained through to Week 52. Thus, secukinumab reduces the negative impact that HS has on employment and work productivity.






