Meeting Summary
Prof Augustin opened the symposium by underlining how the management of patients with moderate-to-severe atopic dermatitis (AD) has become more dynamic over the past 2 years following the approval of the first biologic agent, dupilumab, in 2017. Prof Augustin explained that moderate-to-severe AD is a chronic Type II inflammatory disease that has significant effects on patients’ and caregivers’ lives. The multidimensional disease burden of AD, which includes signs and symptoms that impact physical, mental, social wellbeing, and quality of life (QoL), is proportional to disease severity and lack of disease control. Sustained control of moderate-to-severe AD is essential to limit the burden caused by the disease. In the second presentation, Dr Simpson emphasised the importance of pointing out to each patient that AD is a chronic disease that requires long-term management. When discussing goals and treatment options with each patient, the importance of sustainable disease management should be emphasised. He presented outcomes from recent clinical trials investigating the long-term efficacy and safety of targeted agents in patients with AD. In the final presentation, Dr de Bruin-Weller discussed the importance of real-world evidence when considering treatment options for patients with AD. Real-world evidence for the effectiveness and tolerability of treatments can be gleaned from a number of sources, including registry-based clinical experience, survey data, centre-based clinical experience, and case studies. Consideration of real-world evidence, alongside outcomes from randomised controlled trials, enables selection of the most appropriate treatment option for each patient.
Burden of Moderate-to-Severe Atopic Dermatitis in Adult and Adolescent Patients
Professor Matthias Augustin
AD is a chronic Type II inflammatory disease characterised by dry and scaly eczematous lesions accompanied by intense itching.1 Signs and symptoms of AD include xerosis (dryness), diffuse erythematous patches, oozing papulovesicles, lichenified and excoriated plaques, and frequent and intense pruritus (itch).1
Atopic Dermatitis is a Highly Prevalent Dermatological Condition Affecting Children and Adults Worldwide
AD most often starts in childhood but is highly prevalent in adults.2,3 A recent international survey reported point prevalence (95% confidence intervals) of adult AD in the overall population of 4.9% (4.6–5.2%) in the USA, 3.5% (3.1–3.9%) in Canada, 4.4% (4.2–4.6%) in the European Union (EU), and 2.1% (1.8–2.3%) in Japan.4 AD prevalence was generally lower for males versus females, and decreased with age.4 In addition, 46–55% of adult patients had moderate-to-severe AD, as measured by the Patient-Oriented Eczema Measure (POEM).4
Prof Augustin explained that AD prevalence is higher in children than adults and has been increasing over recent decades. In fact, AD is the most common chronic inflammatory dermatosis in paediatric patients in industrialised countries, with prevalence rates in North America of 8.5–9.1%, the EU of 6.1–10.4%, and Japan of 10.5–16.9%.5-8 Approximately, 10–40% of children with AD have moderate-to-severe disease.7,9-15
The Burden of Atopic Dermatitis is Substantial and Multidimensional
Patients with AD experience a multidimensional burden of disease (Figure 1). In addition to visible skin lesions16 and symptoms that include severe pruritus, pain, and sleep disturbance,17 AD impacts negatively on QoL,17-19 social activities,19 and work productivity.20 AD is associated with mental health disorders, such as anxiety and depression,17,21-24 and a number of comorbid conditions, including skin infections25-29 and Type II inflammatory diseases.17 Indeed, data from AD clinical trials show substantial prevalence of asthma (8.0–42.7%), allergic rhinitis (34.5–50.6%), chronic rhinosinusitis or nasal polyps (2.6%), and food allergy/intolerance (17.4–38.4%) in adult patients with moderate-to-severe AD.30-32
Figure 1: Patients with atopic dermatitis experience a multidimensional burden of disease.
In addition to visible skin lesions and symptoms that include severe pruritus, pain, and sleep disturbance, atopic dermatitis impacts negatively on quality of life, social activities, and work productivity. Atopic dermatitis is associated with mental health disorders such as anxiety and depression, as well as a number of comorbid conditions, including skin infections and Type II inflammatory diseases.
AD: atopic dermatitis; QoL: quality of life.
The Burden of Atopic Dermatitis Increases with Disease Severity
AWARE is an international, cross-sectional, observational study of adults with AD that aims to compare patient-reported burden across disease severity levels.33 Significantly increased measures of pruritus, sleep disturbance, and negative impact on QoL were reported with greater disease severity based on Investigator’s Global Assessment (IGA).33 In addition, the presence of coexisting Type II inflammatory diseases, including asthma and food allergies, also increased with greater disease severity.33 Analysis of work productivity in adult patients showed that even mild AD led to a mean number of 11.2 work/study days missed per year, with even more days missed in patients with moderate disease (24.7 days) and severe disease (45.4 days) (p<0.001 for mild and moderate versus severe AD).34
Additionally, significantly more patients with moderate or severe disease reported that AD influenced their career choice and had a negative effect on their pursuit of education compared with patients with mild AD.34
Children and adolescents with AD also experience a high burden of disease. Data from a German health insurance database showed increased prevalence of skin conditions (i.e., impetigo, vitiligo), Type II inflammatory diseases (i.e., bronchial asthma, allergic rhinitis), arthritis, and mental health disorders (i.e., depression) in patients aged <18 years compared with age-matched counterparts without AD.8 In a Phase III study (N=251), 92% of patients aged 12–17 years with moderate-to-severe AD (n=230) had a self-reported history of ≥1 coexisting Type II inflammatory disease at baseline, including allergic rhinitis (65.6%), food allergy (60.8%), asthma (53.6%), hives (28.8%), and allergic conjunctivitis (22.8%).35 In addition, QoL and social development were significantly impacted in this patient population, with a high proportion of patients reporting affected sleep, feelings of embarrassment and self-consciousness, and a negative impact on clothing choices, leisure activities, and social interactions, including friendships.35 As with adults, the impact on QoL in adolescent patients with AD increases with greater disease severity, as does the impact on caregiver QoL and the number of missed school days.36 This high burden experienced by children and adolescents with AD highlights a need for effective early intervention for this condition.
What are the Unmet Needs in Patients with Atopic Dermatitis?
Disease control is of great importance in patients with AD; inadequate control of AD is common and is associated with increased disease burden. The rate of inadequate disease control significantly increases with AD severity: 45.1% in patients with mild disease; 64.2% of patients with moderate disease; and 84.9% of those with severe disease.37 Many patients report dissatisfaction with commonly available AD treatments; adult patients in the USA reported low satisfaction with topical treatments, systemic immunosuppressants, and phototherapy, with 48.1% of survey respondents ‘extremely’ or ‘very’ dissatisfied.38 In the EU, systemic immunosuppressants are commonly used to treat AD. These agents are associated with multiple risks that can contribute to patient burden, including lymphomas and other malignancies, osteoporosis, diabetes, glaucoma, infections such as eczema herpeticum, and hospitalisations.39 In summary, there is an ongoing need for a safe and effective targeted treatment that provides sustained disease control for patients with moderate-to-severe AD.
Sustainable Management of Moderate-to-Severe Atopic Dermatitis Using Targeted Therapies: Emerging Data from Clinical Trials
Doctor Eric Simpson
Dr Simpson highlighted the importance of achieving sustained management of AD and the ongoing requirement for therapies that are safe and effective for both short and long-term use. He emphasised that the first step to sustainable management of AD is to talk to the patient about their treatment goals and preferences. Every patient is different, but the patient needs to realise that AD is a chronic disease that will require long-term treatment.
There is a paucity of data on the long-term use of systemic immunosuppressive drugs for the treatment of AD. In some countries, there are limits on the length of administration for some agents, for example in Japan the limit for cyclosporine (CsA) use is 8–12 weeks.40 Guidelines published by the European Academy of Dermatology and Venereology (EADV) recommend cessation of CsA after 2 years, with careful monitoring for potential severe side effects.41
Recent advances in immunology research are driving the development of novel systemic therapies for AD, and a number of novel agents are now in Phase II and III clinical trials. Given the need for long-term AD treatment, Dr Simpson focussed on novel therapies with available evidence for long-term efficacy and safety. Two drugs were discussed: the approved therapy dupilumab, and the investigational therapy nemolizumab.
Dupilumab is a fully human IL-4 receptor α monoclonal antibody that potently inhibits signalling of IL-4 and IL-13, leading to decreased numbers of Type 2 cytokines implicated in numerous allergic diseases ranging from asthma to AD.42,43 Dupilumab is the first approved biologic agent that targets Type II inflammation in patients with AD and is approved for the treatment of moderate-to-severe AD in adults and patients aged 12–17 years.42 Efficacy and safety data for 52 weeks’ treatment in a Phase III trial, as well as efficacy and safety data for 76 weeks’ treatment in an open-label extension study, are available for dupilumab.31,43
Nemolizumab is an investigational drug that targets signalling pathways involved in pruritus via inhibition of IL-31.30 Data from a 64-week open label Phase II trial are available in a limited number of patients.44 A Phase III nemolizumab clinical trial is currently recruiting.45
Available Evidence for Sustainable Management of Atopic Dermatitis with Dupilumab
In the 1-year, randomised, double-blind, placebo-controlled CHRONOS study, treatment with dupilumab demonstrated significant and sustained improvements versus placebo in the proportion of patients achieving 75% improvement from baseline using the Eczema Area and Severity Index (EASI-75) at Week 16 that were sustained until Week 52.43 In addition, dupilumab treatment resulted in rapid and sustained improvements in itch and sleep loss,46 and a lower proportion of dupilumab-treated patients required rescue medication versus those who received placebo through Week 52.43 Dupilumab was well tolerated during and up to 52 weeks of treatment, with nasopharyngitis, injection-site reactions (mainly pain and redness), and conjunctivitis comprising the main adverse events (AE) of interest.43 Dr Simpson stated that the majority of conjunctivitis cases reported in dupilumab-treated patients were mild-to-moderate in severity and responded to treatment with anti-inflammatory agents. Furthermore, the incidence of conjunctivitis did not increase over time. The incidence of non-herpetic skin infections in CHRONOS was lower in the groups receiving dupilumab (11% and 8%) than in the placebo group (18%).43
An open-label extension study is currently assessing additional long-term safety and efficacy of dupilumab in adults with mild-to-moderate AD. This is a heterogeneous patient population, enrolling patients who had participated or been screened in 13 prior dupilumab AD studies (3 Phase Ib studies, 5 Phase II studies, and 5 Phase III studies), and includes patients who originally received placebo.31 By Week 76, from the total study population of 1,491 patients who received at least 1 dose of dupilumab, 1.8% of patients had discontinued dupilumab.31 There were rapid and sustained improvements in mean EASI score through Week 76 in both dupilumab-naïve and dupilumab-retreated patients (Figure 2A).31 There were also improvements in AD signs and symptoms and patient QoL at Weeks 52 and 76 with dupilumab treatment (Figure 2B).31 Over 76 weeks, the dupilumab safety profile was consistent with previous studies, including CHRONOS.31,43
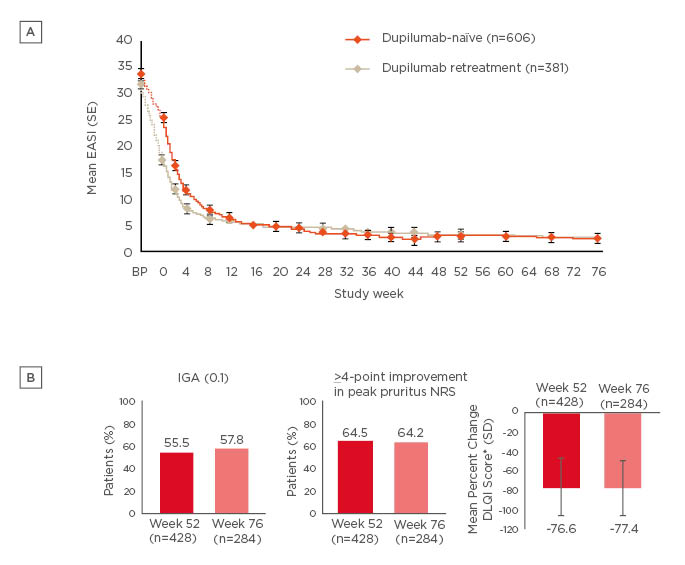
Figure 2: Sustained improvements in (A) mean EASI score over 76 weeks†, and (B) signs, symptoms, and quality of life at Week 52‡ and Week 76¶ with dupilumab treatment in an open-label extension study.
†Efficacy analyses were performed including all patients who reached the respective timepoint or would have reached that timepoint had they not discontinued earlier. Sensitivity analyses (e.g., multiple imputations) were consistent with efficacy in all observed patients, suggesting no bias on treatment outcomes due to patient withdrawal.
‡Efficacy at Week 52 observed for Week 52 cohort, which included all patients enrolled at least 53 weeks prior to data cutoff (accounting for ±1 week visit window).
¶Efficacy at Week 76 observed for Week 76 cohort, which included all patients enrolled at least 77 weeks prior to data cutoff (accounting for ±1 week visit window).
*DLQI was assessed every 12 weeks with the last timepoint for this analysis at Week 48.
DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; IGA: Investigator’s Global Assessment; NRS: numeric rating scale; SD: standard deviation; SE: standard error.
Both figures adapted from Deleuran et al.31
Dr Simpson presented a pooled analysis of safety data from seven Phase II and Phase III studies (n=1,841 for all dupilumab doses combined), and confirmed that dupilumab is not an immunosuppressant, with the overall number of treatment-emergent infections comparable to those observed in patients who received placebo (n=1,091).47 Skin structure and soft tissue infections (risk ratio: 0.44; p<0.001) and clinically important herpes viral infections (eczema herpeticum and herpes zoster) (risk ratio: 0.31; p<0.01) were less common with dupilumab compared with placebo, respectively.47
In addition to clinical trials in adults, dupilumab has also been investigated in adolescent patients. The coprimary endpoints in a Phase III monotherapy study48 were both met, with dupilumab (300 mg every 4 weeks [n=84] or 200/300 mg every 2 weeks [n=82]) treatment significantly improving IGA and EASI-75 response rates at Week 16 compared with placebo (n=85) in patients aged 12–17 years.49 In addition, the dupilumab safety profile in the adolescent population was consistent with that observed in adult patients.49 Consequently, in an open-label extension to the Phase III study that enrolled 36 adolescent patients with moderate-to-severe AD, sustained improvement in EASI-75 was observed in patients treated with dupilumab through Week 52.50 In addition, there was sustained improvement in mean peak pruritus numeric rating scale (NRS) score over the same timeframe, with a 66% reduction from baseline.50 The dupilumab safety profile in this open-label extension cohort was consistent with a previous Phase IIb study, and there were no treatment discontinuations resulting from AE.50
Available Evidence for Sustainable Management of Atopic Dermatitis with Investigational Therapies
Nemolizumab is a humanised monoclonal antibody against IL-31 receptor α that binds to IL-31 receptor α on a number of cells, including neurons, to inhibit IL-31 signalling, which may alleviate pruritus.30 As it is the only investigational agent in AD currently with long-term (≥52 weeks) data available, Dr Simpson provided an overview of outcomes from a nemolizumab Phase II clinical trial. In the two-part study, three separate dosing regimens of nemolizumab resulted in improved signs and symptoms of AD versus placebo at Week 12.30 A total of 191 nemolizumab-treated patients transitioned from the placebo-controlled phase of the study to the open-label phase.44 During the open-label phase, 31.4% of nemolizumab-treated patients discontinued treatment by Week 64, leading to concerns that the remaining population may be those that respond best to this treatment. At Week 64, improvements in pruritus visual analogue scale score and mean EASI score were observed with all nemolizumab dosing schedules, although exacerbation of AD was reported in 21–28% of patients.44 Nemolizumab was generally well tolerated and the safety profile in the 52-week long-term extension was comparable with that observed in the placebo-controlled period; the most common treatment-related AE were nasopharyngitis, AD exacerbation, increase in blood creatinine phosphokinase, upper respiratory tract infection, peripheral oedema, and injection-site reactions.44
Advances in the Management of Moderate-to-Severe Atopic Dermatitis in the Real World
Doctor Marjolein de Bruin-Weller
In the final symposium presentation, Dr de Bruin-Weller explained the importance of real-world evidence when considering treatment options for patients with AD. Patient populations in randomised, controlled trials differ from those seen in the clinic; for instance, they are generally more motivated and compliant with treatment than patients seen in real-world practice. In addition, physician time and resources are likely to be more limited in the ‘real world’ compared with the clinical trial environment. Real-world evidence for the effectiveness and tolerability of treatments can be gleaned from a number of sources, including registry-based clinical experience, survey data, centre-based clinical experience, and case studies.
Registry-Based Clinical Experience in Atopic Dermatitis
Data analyses from registries, especially prospective registries, are very useful for assessing the long-term efficacy and safety of treatments as they are used in daily clinical practice. There are a number of AD registries worldwide and Dr de Bruin-Weller provided an overview of three European-based registries: EUROSTAD,51 TREATgermany,52,53 and BioDay.54
EUROSTAD is a 5-year, observational, industry-sponsored study being performed in 10 European countries that is investigating patient characteristics and disease management in 308 adult patients with mostly moderate-to-severe AD.51 At enrolment, 53.5% of patients were IGA 3 and 31.6% were IGA 4; the mean EASI score was 16.3. The most commonly prescribed systemic therapy at enrolment was CsA (40.5% of patients), with 23.3% of patients receiving methotrexate, 19.0% receiving corticosteroids, and 18.3% receiving dupilumab.51 Outcomes from EUROSTAD are not yet available but are awaited with great interest.
TREATgermany is a long-term, prospective, industry-sponsored registry investigating the use, effectiveness, and safety of treatments for moderate-to-severe AD in >30 centres in Germany.52,53 This registry is an initiative of the German Society of Dermatology (DDG) and is academia-led. Clinicians, and their patients with moderate-to-severe AD, complete assessments every 3–6 months for ≥2 years. In patients enrolled by 2014 (i.e., prior to the availability of dupilumab) (n=78), CsA was the most commonly prescribed systemic treatment and was received by 33.3% of patients.53 In patients who were prescribed CsA (n=35), EASI-75 was achieved by 34% of patients at Weeks 12–24. Over the same time frame, there was no significant impact of CsA treatment on POEM or Dermatology Life Quality Index (DLQI) scores.53 A second analysis, performed on data collected from June 2017 to June 2019, included real-world usage of dupilumab. Patients who received dupilumab showed mean reduction in EASI score at 12 weeks of 74.2% (n=105), which was stable through Week 24 (n=53). In addition, EASI-75 was achieved in 57.1% of patients by Week 12 and in 51.9% of patients at Week 24.55 Improvements in AD symptoms (measured via POEM) and QoL (measured via DLQI) were achieved by Week 12 (both p<0.001 versus baseline) and maintained at Week 24.55,56 The dupilumab safety profile in this real-world data analysis was consistent with clinical trial data: conjunctivitis was the most frequently reported AE (reported by 13.3% of patients at Week 12 and by 29.6% of patients at Week 24).56
The BioDay registry is a prospective, academia-led, industry-sponsored registry involving four university hospitals and seven regional hospitals in the Netherlands that commenced in January 2018. Initial results in 138 patients reported dupilumab efficacy consistent with that demonstrated in clinical trials, with mean reduction in EASI score from baseline of 73.1% and EASI-75 achieved by 61.7% of patients at Week 16.54 Improvements in pruritus NRS and POEM were also consistent with those observed in the CHRONOS and CAFE clinical trials.55 In a ‘responder’ algorithm developed by the investigators, 89.0% of patients achieved at least 1 of the following after 16 weeks treatment with dupilumab:54
- EASI-75
- pruritus NRS ≥4-point improvement
- DLQI ≥4-point improvement
In this patient population, in which 64.5% of patients reported a history of allergic conjunctivitis at study baseline, 34.0% reported allergic conjunctivitis following dupilumab treatment. A total of 20.0% of patients experienced moderate-to-severe conjunctivitis that required treatment with anti-inflammatory agents.54
Centre-Based Clinical Experience in Atopic Dermatitis
Centre-based clinical studies provide outcomes from daily clinical practice for individual countries or regions. Dr de Bruin-Weller presented centre-based clinical experience from three separate studies, of which two were retrospective (performed in Italy [N=109]57 and France [N=241],58 respectively) and one prospective (performed in the Netherlands [N=95]).59 Differences in baseline characteristics and outcome measurements make cross-study comparisons difficult, but in each study dupilumab treatment resulted in rapid improvement in EASI and DLQI score.57-59
Survey Data in Atopic Dermatitis
Data from RELIEVE-AD, a prospective, longitudinal patient survey (N=674) conducted in the USA, have recently been made available. Patients complete a survey prior to receiving dupilumab and at Months 1, 2, 3, 6, 9, and 12 post-treatment commencement. In a 6-month interim analysis, the proportion of patients who were ‘very’ or ‘extremely’ satisfied with their AD treatment increased from 2.9% at baseline to 57.8% at Month 1, and 70.9% at Month 6 (both p<0.001 versus baseline).38 The proportion of patients reporting use of concomitant systemic corticosteroids was 34.7% at baseline, 7.8% at Month 1, and 9.2% at Month 6.38 The proportion of patients reporting use of concomitant AD therapies (excluding dupilumab) from ≥3 drug categories was 13.1% at baseline, 1.7% at Month 1, and 1.5% at Month 6 (both p<0.001 versus baseline). Furthermore, the proportion of patients without any concomitant AD therapy increased from 12.8% at baseline to 39.6% at Month 1 and 41.3% at Month 6 (both p<0.001 versus baseline).38
Case Studies in Special Populations
Dr de Bruin-Weller highlighted the importance of considering the efficacy and safety of novel treatments in special populations that are often excluded from clinical trials, for example patients with chronic hepatitis B virus (HBV), renal failure, or HIV. Case studies can be of value when considering the use of novel treatments in such populations. Dr de Bruin-Weller discussed a case report of two patients with AD and HBV who received dupilumab whilst receiving concomitant therapy for HBV.60 Prior to treatment with dupilumab, AD signs and symptoms were uncontrolled in both patients despite topical or systemic AD treatment. Following treatment with dupilumab, both patients experienced good control of AD. Neither patient reported AE associated with dupilumab. In addition, both patients had normal liver function and an undetectable HBV load.60
Indirect Treatment Comparisons in Atopic Dermatitis
In the absence of head-to-head clinical trial data, indirect comparisons using logistic regression analysis can be used to predict the ‘responder’ rate for a comparator that was not originally tested within a study. The logistic regression model accounts for differences in study populations, including adjustments for baseline characteristics.
As there are no head-to-head comparisons for dupilumab versus ‘standard of care’ in the setting of moderate-to-severe AD, an indirect comparison analysis has been performed using data from patients treated with CsA in daily practice at University Medical Center Utrecht (N=57) and those treated with dupilumab (every 2 weeks) in the Phase III CHRONOS study (N=106). This analysis showed greater numbers of EASI-75 responders with dupilumab versus CsA in both data sets.61
Conclusion
In summary, there is a growing body of real-world evidence for the effectiveness of dupilumab treatment in patients with moderate-to-severe AD. Use of dupilumab in real-world clinical practice has been shown to improve AD signs, symptoms, and patient QoL, all in a manner consistent with clinical trial observations. The dupilumab safety profile in real-world studies is consistent with evidence from randomised controlled trials. There are reports in some real-world cohorts of increased incidence of conjunctivitis compared with clinical trials; conjunctivitis cases rarely led to treatment discontinuation and occurred more frequently in patients with a history of this condition. Reports from other registries, surveys, centre-based studies, and case studies will further our understanding of real-world outcomes associated with the use of dupilumab and other novel systemic therapies. Going forward, there is a need for collaboration across registries because sharing data will further advance our understanding in this therapy area and ensure optimal outcomes for patients.







