Meeting Summary
Prof Reich outlined the new understanding of psoriasis pathogenesis, with IL-23 rather than IL-12 considered the pivotal cytokine pathway. This understanding, along with new therapeutic agents, suggests that complete clearance is becoming a realistic treatment goal for patients.
Prof Iversen gave a detailed description of the pathogenesis of psoriasis. Psoriasis was previously thought to be driven by Th1 cells, but the key driver is now believed to be the IL-23/Th17 pathway. In a newly understood intermediate step, immature T cells develop into either inducible or regulatory T cells; the inducible Th17 cells mature into either pathogenic or non-pathogenic T cells, differentiation is dependent on IL-23 levels. Prof Iversen described findings that suggest IL-12 may have anti-inflammatory properties. This cytokine model may explain the different effects of drugs that target IL-12 and IL-23 versus those that target IL-23 alone.
Prof Reich and Prof Bachelez presented key clinical data on new IL-23-targeted therapeutic agents. The VOYAGE 1 study with guselkumab found Psoriasis Area Severity Index (PASI) 90 rates of 81.1% at Week 100 and PASI 100 rates >49.0%.1 The reSURFACE trials with tildrakizumab demonstrated lower PASI 90 and PASI 100 response rates than VOYAGE 1, but, again, responses were durable and the agent was well-tolerated.2 UltIMMa 1 and 2 were replicate studies that compared the IL-23 inhibitor risankizumab with the IL-12 and IL-23 inhibitor ustekinumab. At Week 52, PASI 90 response rates were 82% for risankizumab, 78% in the group switched to risankizumab after placebo, and 44% for those on ustekinumab.3 This suggested that blocking IL-23 alone is superior to blocking both IL-12 and IL-23. The response to risankizumab was stable and durable; the safety profile was comparable to the comparator ustekinumab. IMMvent4 and IMMhance5 demonstrated robustness of response to risankizumab among patients who had failed prior therapies. The speakers and the audience concluded that these early trials suggest that the IL-23 inhibitors are an attractive new class of agents for the treatment of psoriasis.
Are We in a Psoriasis Evolution or Revolution?
Professor Kristian Reich
The world of psoriasis therapy is expanding rapidly with new therapeutic groups, biosimilars, and small molecules, explained Prof Reich. For decades, cold tar, phototherapy, and methotrexate were the mainstays of treatment. Methotrexate is still used, but has only a 35% PASI 75 response rate.6 The first treatment revolution was the introduction of TNF-α blockers, such as infliximab, which had a 58% PASI 90 response rate at 24 weeks in the EXPRESS study.7 More recently, IL-17 inhibitors showed strong efficacy in psoriatic arthritis, and PASI 90 and PASI 100 response rates 20% higher than with methotrexate (their introduction was another evolution). IL-23 inhibitors make up the third major group of targeted therapy, and the question raised was whether their arrival is an evolution or revolution.
According to the historical model of psoriasis,8 activated myeloid dendritic cells release IL-12, which activates Th1 cells, and also IL-23, which activates Th17 cells. The Th cells release cytokines that activate keratinocytes and bring about the phenotype of psoriasis. Prof Reich said it is becoming clear that the IL-23 pathway drives the development of psoriasis, rather than the IL-12 pathway.
The p40 subunit is common to both IL-12 and IL-23 (Figure 1); drugs that inhibit p40 block both IL and are effective psoriasis treatments,9 said Prof Reich, but the inhibition of IL-23 is the more probable mechanism rather than that of IL-12. A study in 201010 demonstrated that p40 (IL-12Rβ1) is elevated in psoriasis, as is p19 (IL-23R) but not p35 (IL-12Rβ2). Experimental work on human skin biopsies, comparing psoriatic and normal skin, found increased expression of IL-23 p19 and p40 in psoriatic skin and suggested that IL-23 plays a more dominant role in psoriasis than IL-12.11
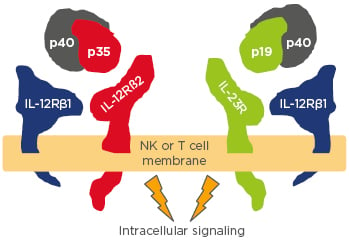
Figure 1: The rationale for targeting IL-12 and IL-23 in psoriasis.
NK: natural killer cell.
Adapted from Benson et al.9
The pathophysiology of psoriasis is now thought to involve a feed forward response by which the immune system activates the epidermis and a feedback response is delivered from the epidermis to the immune system.12 This concept, along with the introduction of new therapies, is changing treatment goals. Eight years ago, Prof Reich believed that long-term skin clearance could never be a realistic treatment goal; it is now becoming technically possible and treatment goals need to be adapted. The traditional approach, scaling up treatment over months or years, may be the worst approach from an immunological point of view. Furthermore, treatments may eventually be introduced that modify the disease in the same way as antirheumatics modify rheumatoid arthritis. It could mean that patients remain free of psoriasis for prolonged periods of time after treatment has stopped. There might be markers to indicate that an individual patient will develop psoriasis; treatment could prevent the disease from ever breaking out. There is progress towards this vision; Prof Reich said the management of psoriasis is undergoing an evolution that might turn into a revolution.
Take Home Message
It is becoming clear that the IL-23 pathway plays a more dominant role in the development of psoriasis than the IL-12 pathway. Treatments that inhibit both pathways may owe their efficacy to inhibition of the IL-23 pathway. The new IL-23 inhibitors are contributing to new goals in psoriasis therapy: long-term skin clearance may now be a realistic aim of treatment.
IL-23: A Master Regulatory Cytokine in Psoriasis?
Professor Lars Iversen
An understanding of the pathogenesis of psoriasis is essential to distinguish between treatments with distinct modes of action, Prof Iversen stated. Psoriasis patients have a genetic predisposition, which does not cause disease unless triggered. A streptococcal infection, for example, could activate keratinocytes that release cytokines that activate dendritic cells, which drive the disease process.8
Activated dendritic cells migrate to lymph nodes and induce the maturation of immature Th cells. The cytokine milieu will determine the direction in which the Th cells differentiate. IL-23 drives the maturation of Th cells into Th17 cells, a key driver in psoriasis. Th17 cells release cytokines, such as IL-17, which causes the phenotypical changes in the skin seen in psoriasis.13 Many recent drug developments in psoriasis have therefore targeted IL-17 and results have been promising. Secukinumab and ixekizumab are approved inhibitors of IL-17A. Brodalumab, also approved, targets the receptor that signals IL-17 A, F, C, and E. Bimekizumab, currently undergoing clinical trials, is an antibody directed against IL-17A and IL-17F.
Approved IL-23 inhibitors include guselkumab and tildrakizumab. Phase III trial data are available for risankizumab, but the drug is not yet approved. Mirikizumab is in earlier clinical trial development. As previously mentioned, psoriasis was previously thought to be driven by Th1, but the key driver is now believed to be the IL-23 Th17 pathway.14,15 Naïve T cells exposed to IL-23 differentiate into Th17 cells and release signature cytokines, such as IL-17A, IL-17F, and IL-22.
It is now known that there is an intermediate step in T cell maturation. The interaction between dendritic cells and immature T cells is regulated by surrounding concentrations of TGF-β and IL-6. High concentrations of TGF-β favour the development of regulatory T cells; low concentrations of TGF-β and IL-6 favour development into inducible Th17 cells.14,15
The subsequent differentiation of inducible Th17 cells is driven by IL-23.14,15 As illustrated in Figure 2, high concentrations of IL-23 drive the maturation of inducible Th17 cells into pathogenic Th17 cells. These pathogenic cells release the cytokines that result in psoriasis. However, in conditions of no IL-23, or very low concentrations, inducible Th17 cells mature into non-pathogenic Th17 cells that release cytokines such as IL-17 and IL-10. These cytokines may be anti-inflammatory and have a protective effect at mucous and possibly cutaneous membranes. In this way, IL-23 concentrations determine whether the mature Th17 cells are pathogenic or non-pathogenic.
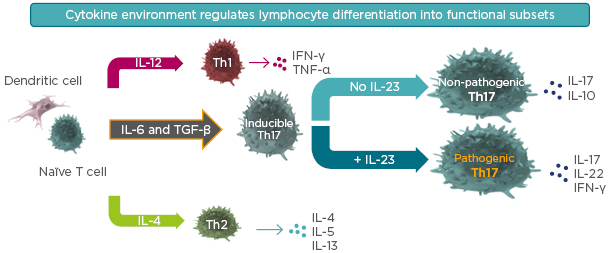
Figure 2: Inducible Th17 differentiation in the presence or absence of IL-23.
Selective targeting of IL-23 helps preserve protective, non-pathogenic Th17 cells that produce IL-17 involved in mucosal defense and barrier tissue integrity.
TGF-β: transforming growth factor β.
Adapted from Leung et al.14 and Zhu et al.15
This scheme may explain why agents that target both IL-12 and IL-23 may have lower efficacy than those that target IL-23 alone, Prof Iversen explained. Ustekinumab targets the p40 subunit and inhibits both IL-12 and IL-23, while risankizumab blocks IL-23 alone. A Phase II, 48-week trial of risankizumab versus ustekinumab16 randomly assigned a total of 166 patients to receive one of three doses of risankizumab (a single 18 mg dose at Week 0 or 90 or 180 mg [according to body weight] doses at Weeks 0, 4, and 16) or ustekinumab (45 or 90 mg [according to body weight] at Weeks 0, 4, and 16). At Week 12, the percentage of patients with a ≥90% reduction in the PASI score was 77% (64 of 83 patients) for risankizumab (90 mg and 180 mg groups, pooled), compared with 40% (16 of 40 patients) for ustekinumab (p<0.001). Risankizumab was associated with clinical responses superior to those associated with ustekinumab.
Potential explanations for this difference include drug affinity or dosing, but Prof Iversen stated that it is also possible that IL-12 (blocked by ustekinumab but not risankizumab) has a beneficial role. A recently published study17 listed the IL-12 family according to proinflammatory profile. The study suggested that IL-23, which is part of this family, is more proinflammatory than IL-12. IL-12 has some anti-inflammatory properties, and it may be beneficial to maintain IL-12 during treatment for psoriasis. Other research supports the inhibitory role of IL-12 on inducible Th17 cells.14,15,18
Prof Iversen also discussed the difference between targeting IL-23 and IL-17. IL-17 is the downstream driver of phenotypical changes in the skin, and IL-17A inhibitors are effective in the treatment of psoriasis. However, inhibition of IL-17A may have the potential for a higher risk of adverse events or infections compared with IL-23 inhibitors.19,20 Mucocutaneous candidiasis is seen more often with IL-17A inhibitors than with IL-23 inhibitors.14,15 The effect is not large or proven, but Prof Iversen commented that it may be because IL-17 inhibitors block the IL-17 produced by non-pathogenic as well as by pathogenic Th17 cells. The contribution of IL-17 from non-pathogenic Th17 cells, left intact by IL-23 blockers, may have a beneficial effect.
Discussion on the pathogenesis of psoriasis should focus on immune modulation rather than immune suppression. Prof Iversen said IL-23 is a master regulatory cytokine in the pathogenesis of psoriasis. It regulates the differentiation of inducible Th cells into pathogenic and non-pathogenic Th17 cells. Inhibition of IL-23 reduces the pathogenic Th17 cell population and potentially results in prolonged downregulation of immune activation.
Take Home Message
Discussion of the pathogenesis of psoriasis should focus on immunomodulation rather than immunosuppression. The differentiation of immature T cells into either regulatory T cells or inducible Th17 cells is regulated by surrounding concentrations of TGF-β and IL-6. Concentrations of IL-23 then drive the maturation of inducible Th17 cells into either pathogenic or non-pathogenic Th17 cells. High concentrations of IL-23 favour the development of pathogenic Th17 cells that release the cytokines that result in psoriasis.
The cytokine IL-12 is involved in the differentiation of immature cells into Th1 cells. Th1 cells produce IFN-γ, which is a negative regulator of inflammatory cytokine production by Th17 cells, γδ T cells, and innate lymphoid cells. Thus, preserving the IL-12 pathway and inhibiting IL-23 alone could lead to better treatment outcomes compared with combined blockade of IL-12 and IL-23.
Inhibition of Il-17, the downstream driver of phenotypical changes in the skin, may have the potential for a higher risk of adverse events compared with IL-23 inhibitors. This could be because IL-17 inhibitors block the IL-17 produced by non-pathogenic as well as by pathogenic Th17 cells. Selective targeting of IL-23 helps preserve protective, non-pathogenic Th17 cells that produce IL-17 involved in mucosal defense and barrier tissue integrity.
IL-23 Inhibition: The Potential For Durable Disease Control?
Professor Kristian Reich
Guselkumab
The first IL-23 inhibitor to be approved was the monoclonal antibody guselkumab. The VOYAGE 1 study1 compared its efficacy and safety with the TNF-blocker adalimumab. Patients were randomised to receive 100 mg guselkumab (Weeks 0 and 4, then every 8 weeks, n=329); or placebo (Weeks 0, 4, and 12, switching to guselkumab at Week 16 and 20, then every 8 weeks, n=174); or adalimumab (80 mg Week 0, 40 mg Week 1, then 40 mg every 2 weeks until Week 47, n=334). At Week 16, guselkumab was superior to both adalimumab and placebo (73.3% versus 49.7% versus 2.9% of patients achieved PASI 90; p<0.001), respectively. The placebo group was then switched to guselkumab for the remainder of the study. At Week 52, 78.9% of this group had achieved PASI 90, similar to the 80.1% of patients receiving guselkumab from the beginning of the study and substantially higher than the 50.5% of those receiving adalimumab.21
The response seen in VOYAGE 11 was maintained. From Week 52, all patients were switched to guselkumab and at Week 100, PASI 90 rates were similar across all groups, ranging from 81.1–82.3%; PASI 100 rates were >49.0%.21 Prof Reich stated that the curves are flat and represent long-term, stable, safe control of psoriasis, adding that the data in VOYAGE 1 are conservative. The non-responder imputation of 72.3 for PASI 90, and 43.2 for PASI 100, represents longevity of response. As long as patients remain on the drug, the response is stable over a 2-year period.
A common theme among IL-23 inhibitors is that the disease takes longer to return than the pharmacology would predict when the drug regimen is stopped in responding patients. In the withdrawal arm of VOYAGE 2, the last dose of guselkumab was given at Week 20. At Week 48, 36.8% (67 out of 182) of patients still had a PASI 90 response.22
Findings from VOYAGE 2 demonstrate that treatment with guselkumab reduced cytokines in peripheral blood to levels similar to those found in healthy controls.22 Those who maintain a PASI 90 response after drug withdrawal had continued suppression. Loss of response (<PASI 75) was associated with increased levels of serum IL-17A, IL-17F, and IL-22.
Early results suggest the safety of IL-23 inhibitors is encouraging. Serious infections are the most common adverse events, with 1.03 reported per 100 patient-years in from Week 0–48 of giving guselkumab,21 which is probably similar to the background rate suggested Prof Reich.
Tildrakizumab
Tildrakizumab targets the p19 subunit of IL-23. The reSURFACE 1 trial randomised patients to tildrakizumab 200 mg, 100 mg, or placebo. In reSURFACE 2, patients were randomised to the same three groups plus an extra arm receiving 50 mg etanercept.2 In reSURFACE 1 at Week 12, >60% of patients in both tildrakizumab arms achieved PASI 75 compared with 6% of those on placebo. Patients on placebo were then switched to tildrakizumab arms, and at 28 weeks >70% of patients in all arms achieved PASI 75. Results from reSURFACE 2 were similar.2
Prof Reich highlighted data from a pooled analysis of reSURFACE 1 and 2, plus P05495, which showed that 37% and 39% of patients on 200 mg or 100 mg of tildrakizumab achieved PASI 90 at Week 12, and 54% and 58% at Week 28, respectively.2 Both PASI 90 and PASI 100 response rates were slightly lower than with guselkumab. Drug affinity is one potential explanation; a simple dose effect is unlikely because response rates were similar with both doses of tildrakizumab.
Responders to tildrakizumab who achieved PASI 75 at Week 28 maintained the effect off-drug. Tildrakizumab responders were switched to placebo at Week 28; at Week 64, 57% of patients on 200 mg tildrakizumab and 49% of patients on 100 mg remained PASI 75 responders.2,23 The reSURFACE studies found tildrakizumab shared the same safety profile as other IL-23 inhibitors and was comparable to etanercept.2
Risankizumab: UltIMMa-1 and UltIMMa-2
Professor Hervé Bachelez
The Phase III trial UltIMMa-1 compared risankizumab and ustekinumab; UltIMMa-2 is a replicate study.3 In UltIMMa-1 and 2, patients were representative of clinical practice in terms of demographics and disease severity. Prior biologic use, a surrogate marker of the severity of disease, was unusually high at between 30% and 56% across the various arms.
In both UltIMMa-1 and 2, patients were randomised using a 3:1:1 ratio to receive either 150 mg risankizumab, 45 or 90 mg ustekinumab (as indicated according to bodyweight), or placebo. In the induction phase, treatment was administered at 0 and 4 weeks and the primary response assessment took place at Week 16. At this point, patients who had been on placebo switched to risankizumab. All patients were treated at Week 16 and every 12 weeks thereafter.3
Results at Week 16 showed that, of the patients treated with risankizumab, 75.3% of those in UltIMMa-1 and 74.8% of those in UltIMMa-2 achieved PASI 90, one of the co-primary endpoints. This compares with 4.9% and 2.0%, respectively, of patients on placebo. The other co-primary endpoint, the proportion of patients achieving clear or almost clear status (static Physicians Global Assessment [sPGA] 0–1) in the two trials, was 87.8% and 83.7% with risankizumab, compared with 7.8% and 5.1% with placebo (p<0.001).3
At Week 52, the PASI 90 response was 82% for patients on risankizumab, 78% for those switched to risankizumab after placebo, and 44% for those on ustekinumab. Figure 3 demonstrates the stability of response with risankizumab, and contrasts with the fluctuations in response with ustekinumab. A similar pattern is seen in sPGA 0–1 scores up to Week 52: 56% of patients achieved clear skin at Week 52 (PASI 100 and sPGA 0). Prof Bachelez said the two analyses were remarkably convergent and promising.
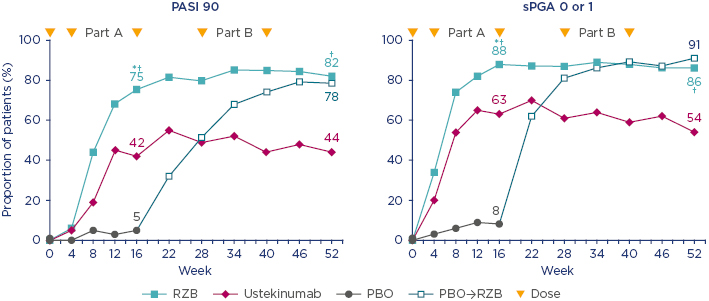
Figure 3: UltIMMa-1: Psoriasis Area Severity Index 90 and static Physicians Global Assessment 0 or 1 through to Week 52 (nonresponder imputation).
*p value for comparison versus placebo; p<0.0001. †p value for comparison versus ustekinumab; p<0.0001.
PASI: Psoriasis Area and Severity Index; PBO: placebo; RZB: risankizumab; sPGA: static Physicians Global Assessment; →: corssover
Adapted from Gordon et al.3
Speed of effect was also notable in these studies. At Week 16, there was a 90% and 92% mean improvement in PASI from baseline among patients on risankizumab in UltIMMa-1 and UltIMMa-2, respectively;3 this effect is compelling since patients had only received two doses of risankizumab at this timepoint.
Patient-reported outcomes mirrored the clinical responses. The proportion of patients receiving continuous risankizumab reported limited-to-no impact on quality of life; the Dermatology Life Quality Index (DLQI) of 0 or 1 was 66% and 67% of patients in UltIMMa 1 and UltIMMa-2 at Week 16 and 75% and 71% at Week 52, respectively, significantly higher than with ustekinumab (p<0.001).3 The same trend is seen on the patient symptom scale, which measures pain, burning, itching, and redness.3 Prof Bachelez said there is no doubt of risankizumab’s superiority over ustekinumab in these data.
Risankizumab had a safety profile comparable to ustekinumab. Drug-related adverse events were balanced across the three arms, and two major adverse cardiovascular events that occurred were considered not related to the treatment by investigators.3 Prof Bachelez acknowledged that the numbers in the trial are not huge, but concluded that the results show the “clear superiority” of IL-23 specific blockage with risankizumab over dual inhibition of IL-12 and IL-23 with ustekinumab.
Risankizumab: IMMvent and IMMhance
Professor Kristian Reich
IMMvent, a head-to-head study comparing risankizumab with adalimumab, found that risankizumab achieved statistically higher PASI 90 and PASI 100 response rates than adalimumab at all time points starting at Week 8.4 Of patients on risankizumab, 72.4% reached PASI 90 at Week 16, compared to 47.4% on adalimumab (p<0.001).
At Week 16, patients with PASI responses between 50 and <90 were rerandomised either to continue on adalimumab or switch to risankizumab. At Week 44, of patients switched to risankizumab, 66% achieved PASI 90, and 40% achieved PASI 100. This compares to 21% and 7%, respectively, of patients who remained on adalimumab (p<0.001).4 Risankizumab is effective and IMMvent found no new safety signal, Prof Reich surmised that these are early results, with small participant numbers, but they look promising.
Previous failure with a biologic is the biggest predictor of suboptimal response to a second biologic. In an integrated analysis of 1,005 patients from Phase III trials: IMMhance,5 UltIMMa-1, and UltIMMa-2,3 PASI response at Week 16 was assessed in subgroups based on past treatment history. In this integrated analysis, 487 patients had nonbiologic systemic therapy experience and 452 had biologic therapy experience. In patients receiving risankizumab, PASI 90 was achieved by 73% (n=168) who previously failed one biologic; and 69% (n=108) who had failed ≥2 biologics, non-responder imputation.24 Prof Reich stated that he had not seen such robustness of response in any other study.
In summary, the IL-23 inhibitors are an attractive class of drugs with risankizumab proving a very promising treatment option. Trial data have yet to be matched in the clinic but Prof Reich said the class looks promising.
Take Home Message
The first IL-23 inhibitor to be approved was guselkumab, which was superior to both adalimumab and placebo in the VOYAGE 1 study. Guselkumab demonstrated long-term, stable, safe control of psoriasis.1 In the reSURFACE 1 and 2 trials, tildrakizumab demonstrated slightly lower PASI 90 and PASI 100 response rates than with guselkumab, but patients who responded to tildrakizumab had relatively long disease control off-drug.2 The UltIMMa-1 and 2 trials found that, at Week 16, 75% patients on risankizumab, reached PASI 90.3 Risankizumab had a safety profile comparable to ustekinumab in these trials and the results demonstrate the superiority of IL-23 specific blockage with risankizumab over dual inhibition of IL-12 and IL-23 with ustekinumab.
Conclusion
Discussion on the pathogenesis of psoriasis should focus on immune modulation rather than immune suppression. New understanding suggests that the IL-23 pathway plays a more dominant role than the IL-12 pathway; IL-23 may be considered a master regulatory cytokine. High concentrations of IL-23 favour the maturation of inducible Th17 cells into pathogenic (rather than nonpathogenic) Th17 cells; these pathogenic cells release the cytokines that result in psoriasis.
Clinical trials have found promising PASI 90 and PASI 100 response rates with the new IL-23 inhibitors. Some work suggests that targeting IL-23 alone is more effective than targeting both IL-12 and IL-23.3 This may be because IL-12 has some anti-inflammatory properties and may have an inhibitory role on inducible Th17 cells.
The new IL-23 inhibitors are contributing to new goals in psoriasis therapy: long-term skin clearance is now a realistic aim. Psoriasis is considered a non-scarring disease, but it tends to reappear in the same site, which supports the idea that it leaves a ‘molecular scar’. Early studies suggest that the skin is populated by memory cells that produce the cytokine profile seen with pathogenic Th17 cells.25 Complete skin clearance, which could eliminate the memory cells, may be important for long-term control of psoriasis. In conclusion, the IL-23 inhibitors are a new class of safe and effective drugs that may help achieve complete skin clearance for many patients with psoriasis.






