Meeting Summary
Vitiligo is an autoimmune skin disorder characterised by acquired distinct white patches of the skin. Currently, limited treatment options are available for vitiligo, and there are no approved systemic therapies.
At the European Academy of Dermatology and Venereology (EADV) Congress in Berlin, Germany, 11th–14th October 2023, Thierry Passeron, Professor and Chair of Dermatology at the Côte d’Azur University, Nice, France, and Head of the Laboratory INSERM U1065 Team 12, Centre Méditerranéen de Médecine Moléculaire (C3M), Côte d’Azur University, Nice, France, presented an oral presentation summarising data from a Phase IIb study of upadacitinib, an oral selective JAK inhibitor, in patients with non-segmental vitiligo (NSV), during the ‘Free Communications II’ session.
Passeron gave an overview of the multicentre, randomised, double-blind, placebo-controlled, dose-ranging study that evaluated the efficacy and safety of upadacitinib in adults with NSV. This article focuses on the clinical efficacy endpoints and the safety profile outcomes presented at the EADV congress. Overall, the study demonstrated the clinical efficacy of upadacitinib at 24 weeks at different doses versus placebo, with improvements in repigmentation over time, up to Week 52, with a favourable safety profile.
Introduction
Vitiligo is an autoimmune skin disorder that causes depigmentation of the skin and/or leukotrichia of the hair, resulting from the loss of melanocytes.1 The global prevalence of vitiligo ranges from 0.5–2.0%, with more than 75% of patients exhibiting NSV, where vitiligo patches are distributed on both sides of the body.2,3
JAK inhibition is a mode of action of interest in vitiligo, as it disrupts the process of immune-mediated melanocyte apoptosis.1 Upadacitinib, an oral selective JAK inhibitor, is currently approved for various immune-mediated inflammatory diseases, including rheumatoid arthritis; atopic dermatitis; psoriatic arthritis; axial spondyloarthritis, including ankylosing spondylitis; ulcerative colitis; and Crohn’s disease.4
Study Design Evaluating Upadacitinib in Non-segmental Vitiligo
In this multicentre, randomised, double-blind, placebo-controlled, dose-ranging study, eligible patients were adults aged 18–65 years with a clinical diagnosis of NSV, baseline Facial Vitiligo Area Scoring Index (F-VASI) of ≥0.5, Total Vitiligo Area Scoring Index (T-VASI) of ≥5, and with no prior use of topical or systemic JAK inhibitors.5
A total of 185 patients were enrolled and randomly assigned to receive a placebo, or upadacitinib at either 6 mg (UPA6), 11 mg (UPA11), or 22 mg (UPA22), once daily for 24 weeks in the placebo-controlled period (referred to as period one; Figure 1).5 During the blinded extension period, patients who had received upadacitinib during period one continued with their respective regimens, while patients who previously received placebo were switched to receive upadacitinib once daily, at either 11 mg or 22 mg, for up to 52 weeks.5
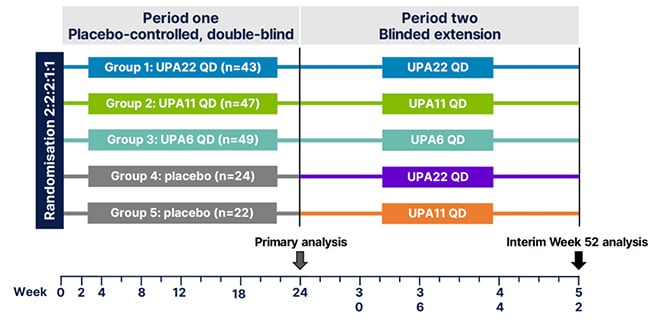
Figure 1: Study design of the Phase IIb, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study.5
QD: once daily; UPA6: upadacitinib 6 mg; UPA11: upadacitinib 11 mg; UPA22: upadacitinib 22 mg.
The treatment groups were comprised of patients with a mean age between 45–48 years, consisting of 71.1–78.6% White, 2.4–9.5% Black, 13.3–15.9% Asian, and 0.0–8.9% from ‘other’ or ’multiple’ races, including Indigenous American or Alaska Native, Native Hawaiian, or Pacific Islander. Passeron reported that patients across all treatment groups had a baseline T-VASI of 21.0–22.3, F-VASI of 1.0–1.2, and 67.3–74.4% reported active vitiligo.
The primary endpoint was percent change from baseline in F-VASI at Week 24, with key secondary endpoints including ≥50% and ≥75% reduction from baseline in F-VASI (F-VASI 50 and F-VASI 75), change from baseline in T-VASI, and ≥50% reduction from baseline in T-VASI (T-VASI 50) at Week 24. These endpoints were followed through the extension period, during which 89.2% (n=165 out of 185) of patients continued, with interim data through Week 52 reported herein.
Efficacy and Safety of Upadacitinib in Non-segmental Vitiligo
Upadacitinib achieved the primary endpoint of percent change from baseline in F-VASI at Week 24, with improvements in both UPA11 (-35.6%) and UPA22 (-34.0%) versus placebo (-14.4%; p≤0.01 and p≤0.05, respectively; using a mixed-effects model for repeated measures).
Patients achieved greater response rates for F-VASI 50 and F-VASI 75 with UPA11 (38.3% and 19.1%, respectively) and UPA22 (39.5% and 14.0%, respectively), compared with placebo (10.9% and 2.2%, respectively; nominal p≤0.05 for both doses and endpoints, based on non-responder imputation with multiple imputation analysis). Additionally, response rates for T-VASI 50 were greater with UPA22 (11.6%) compared with placebo (2.2%; nominal p≤0.05) at Week 24.
Extension Period Outcomes Through Week 52 as Observed
Based on as observed analysis, patients treated with upadacitinib continued to show a reduction in F-VASI and T-VASI through Week 52. Percent change from baseline in F-VASI at Week 52 for UPA6, UPA11, and UPA22 was -53.7%, -60.7%, and -69.8%, respectively. Patients who transitioned from placebo to UPA11 or UPA22 also demonstrated a continued reduction in F-VASI (-51.7% and -57.5%, respectively). The proportion of patients achieving F-VASI 50 was 47.8%, 63.6%, and 82.4% with UPA6, UPA11, and UPA22, respectively. Furthermore, approximately 50% of patients achieved F-VASI 75 with 39.1%, 54.5%, and 41.2% of patients at these respective doses.
Passeron also noted improvements in T-VASI through Week 52 (Figure 2), with nearly a 50% reduction observed in UPA22. Patients who transitioned from placebo to UPA11 or UPA22 at Week 24 also demonstrated reductions in T-VASI (-34.9% and -32.8%, respectively). T-VASI 50 responses were observed in 34.8%, 45.5%, and 41.2% of patients treated with UPA6, UPA11, and UPA22, respectively.
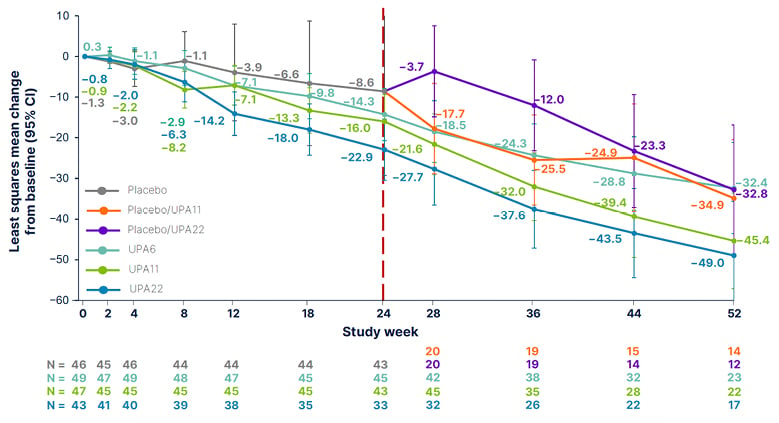
Figure 2: Percent change from baseline in Total Vitiligo Area Scoring Index* through Week 52 over time, as observed.6
*Values are least squares mean (95% CIs), based on ANCOVA using as observed data. Patients who received a placebo in period one were switched to UPA11 or UPA22 in the extension period, in a blinded fashion, per pre-specified randomisation. Data cut-off date of 13 January 2023.
ANCOVA: analysis of covariance; CI: confidence interval; UPA6: upadacitinib 6 mg; UPA11: upadacitinib 11 mg; UPA22: upadacitinib 22 mg.
Safety Profile of Upadacitinib in Non-segmental Vitiligo
Treatment-emergent adverse events were generally similar between upadacitinib and placebo during period one, with the most commonly reported being COVID-19, acne, headache, and nasopharyngitis. Study drug discontinuation due to treatment-emergent adverse events was greater in UPA22 than in other groups. One adjudicated event of non-fatal ischaemic stroke was reported with UPA11 during the extension period, but there were no other adjudicated events, such as venous thromboembolism or gastrointestinal perforation. There was no reported active tuberculosis, or malignancy.
Conclusion
In this Phase II study of adults with NSV, treatment with upadacitinib for 24 weeks resulted in greater improvements versus placebo in clinical outcomes. Observed clinical efficacy continued to improve through Week 52 with upadacitinib treatment. Upadacitinib was generally well tolerated, with no new safety signals identified beyond the known safety profile for upadacitinib. Assessment of upadacitinib as a potential treatment for vitiligo will continue in Phase III trials.






