Presenters: Kristian Reich,1 Xia Li,2 J. Mark Jackson,3 Tania F. Cestari,4 Andrew Blauvelt5
1. Translational Research in Inflammatory Skin Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
2. Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
3. University of Louisville, Division of Dermatology, Louisville, Kentucky, USA
4. Federal University of Rio Grande do Sul, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
5. Oregon Medical Research Center, Portland, Oregan, USA
Disclosure: Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor, Covagen, Dermira, Eli Lilly, Forward Pharma, Fresenius Medical Care, Galapagos, Galderma, GSK, Janssen, Kyowa Kirin, LEO Pharma, Medac, MSD, Miltenyi Biotec, Novartis, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB Pharma, Valeant/Bausch Health, and Xenoport. Li has been an investigator of Eli Lilly and Company. Jackson is an advisor, consultant, researcher, and/or speaker for AbbVie, Janssen, Lilly, Novartis, Ortho, Pfizer, Sanofi/Genzyme, Sun Pharmaceutical Industries, Inc., and UCB. Cestari has received grants, funding, or speaking fees from AbbVie, Janssen, L’Oréal, Pfizer, Sanofi Genzyme, and Vichy Laboratories. Blauvelt has served as a scientific adviser and/or clinical study investigator for AbbVie, Aligos, Almirall, Amgen, Arcutis, Arena, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Leo, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB Pharma.
Acknowledgements: Medical writing assistance was provided by Jennifer Taylor, London, UK. Our thanks are given to the presenters of the sessions and ePosters summarised in this article.
Support: This article was funded by an educational grant from UCB Pharma, who had no input into its content. The views and opinions expressed are those of the presenters/authors.
Citation: EMJ Dermatol. 2021;9[Suppl 6]:2-10.
Meeting Summary
Kristian Reich, Professor of Translational Research in Inflammatory Skin Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, introduced the Phase III BE RADIANT trial in which the efficacy of the IL-17A/F inhibitor bimekizumab (BKZ) was compared with the IL-17A inhibitor secukinumab (SEC) in patients with moderate-to-severe plaque psoriasis. BZK achieved superior levels of complete skin clearance through 48 weeks of treatment, as shown by higher psoriasis area and severity index (PASI) 100 responses compared with SEC. A faster onset of response was also seen with BZK compared to SEC. Results with another IL-17A inhibitor, ixekizumab (IXE), were presented in an ePoster by Li et al. IXE achieved statistically significantly higher response rates at Week 12 compared to the placebo (PBO) in Chinese patients with moderate-to-severe plaque psoriasis. ePosters were presented on four IL-23 inhibitors: guselkumab (GUS), tildrakizumab (TIL), risankizumab (RZB), and mirikizumab (MIR). Reich discussed the VOYAGE 2 Phase III trial, which showed maintenance of response through up to 5 years of continuous GUS treatment in patients with moderate-to-severe psoriasis. J. Mark Jackson, Professor of Dermatology, University of Louisville, Kentucky, USA, presented a post hoc analysis of the reSURFACE 1 and reSURFACE 2 trials and extension periods showing that TIL 100 mg provided durable efficacy through up to 196 weeks of treatment for moderate-to-severe plaque psoriasis, as demonstrated by PASI 75/90/100 and physician’s global assessment (PGA) 0/1. Tania F. Cestari, Federal University of Rio Grande do Sul, Hospital de Clínicas de Porto Alegre, Brazil, reported the results of a Phase III trial in Brazil comparing RZB with methotrexate (MTX) in patients with moderate-to-severe plaque psoriasis. Significantly greater proportions of patients treated with RZB compared with MTX achieved PASI 90 and static PGA (sPGA) 0/1 at Week 28. Andrew Blauvelt, President of Oregon Medical Research Center, Portland, Oregan, USA, presented the Phase III OASIS-1 trial of MIR versus PBO in patients with moderate-to-severe plaque psoriasis. MIR demonstrated superiority compared with PBO according to sPGA 0/1 and PASI 90 at Week 16 and high levels of maintained response were shown at Week 52.
Bimekizumab Efficacy and Safety Versus Secukinumab in Patients with Moderate-to-Severe Plaque Psoriasis: Results from the Phase IIIb Trial BE RADIANT
Kristian Reich
In the late-breaking research sessions, Reich presented BE RADIANT, the first Phase III trial to compare inhibition of IL-17A and IL-17F with inhibition of IL-17A in patients with moderate-to-severe plaque psoriasis.1
IL-17A and IL-17F are both overexpressed in psoriasis and there is increasing evidence that both have a pro-inflammatory role.2,3 IL-17A and IL-17F work together as dimers, including an IL-17A/A homodimer, IL-17A/F heterodimer, and IL-17F/F homodimer. SEC selectively neutralises IL-17A only4 whereas BZK selectively inhibits both IL-17A and IL-17F.5 Reich explained that SEC therefore blocks IL-17A/A and has some inhibitory effect on IL-17A/F but will not block IL-17F/F, in contrast to BZK which inhibits all three cytokines.
BE RADIANT was a 48-week multicentre, randomised, double-blinded trial that compared the efficacy and safety of BZK versus SEC in patients with moderate-to-severe plaque psoriasis.1 Reich outlined the study design, which was a head-to-head comparison of BKZ 320 mg used every 4 weeks (q4w) for the first 16 weeks and then continued as a maintenance dose 320 mg either q4w or every 8 weeks (q8w) for 32 weeks. SEC was used at the approved dose of 300 mg q4w during maintenance and weekly injections at the beginning of therapy. A total of 743 patients were randomised 1:1 to the two treatments. The primary endpoint was PASI 100 response at Week 16. Secondary endpoints included assessment of long-term response with PASI 100 at Week 48 and evaluation of speed of onset with PASI 75 at Week 4.
Reich highlighted the demographics and baseline disease characteristics in the BZK (n=373) and SEC (n=370) groups and noted that they were similar to both previous studies and clinical practice. Average weight was 90.1 kg in the BZK group and 88.8 kg in the SEC group, while average PASI was 20.2 in the BZK group and 19.7 in the SEC group. The Mean Dermatology Quality of Life Index (DLQI) was >10 in both groups (10.8 BZK and 11.3 SEC) showing severe impact of the disease in many patients. And >30% had received biologics before (33.5% of the BZK group and 32.2% of the SEC group).
BE RADIANT demonstrated superior clinical benefit with BZK treatment over SEC treatment, meeting all primary and ranked secondary endpoints. Regarding the primary endpoint, BZK achieved superior levels of complete skin clearance versus SEC at Week 16, with 61.7% of patients in the BZK group achieving PASI 100 compared to 48.9% of patients in the SEC group (p<0.001) (Figure 1). Superiority was maintained at 48 weeks with both 320 mg q8w and 320 q4w BZK maintenance treatment, with 67.0% in the BKZ group achieving PASI 100 compared to 46.2% in the SEC group (p<0.001) (Figure 1).
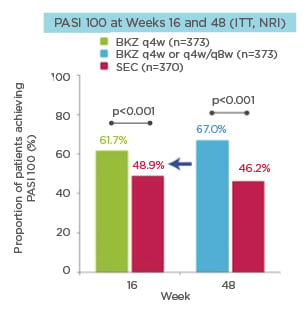
Figure 1: PASI 100 at Weeks 16 and 48 (ITT, NRI).
BKZ: bimekizumab; ITT: intention-to-treat; NRI: non-responder imputation; PASI 100: 100% improvement from baseline in Psoriasis Area and Severity Index; q4w: every 4 weeks; q8w: every 8 weeks; SEC: secukinumab.
These efficacy results were confirmed when the authors analysed only the data for patients that received maintenance beyond Week 16. The efficacy delta in PASI 100 between BZK and SEC remained over time. At Week 48, PASI 100 was achieved by 73.5% of the BZK q4w/q4w group (n=147) versus 48.3% of the SEC group (n=354; p<0.001). Similarly, 66.0% of the BZK q4w/q8w group (n=215) achieved PASI 100 at Week 48 versus 48.3% of the SEC group (n=354; p<0.001). Reich highlighted that the BZK q4w and q8w dosing appeared equally effective in maintaining PASI 100 response over time.
Significant differences were also observed between the two drugs in the proportion of patients achieving PASI 90 at Weeks 16 and 48. At Week 16, 85.5% of the BKZ q4w group (n=373) versus 74.3% of the SEC group (n=370) achieved PASI 90 (nominal p<0.001), while the proportions at Week 48 were 83.6% for BKZ q4w or q4w/q8w (n=373) and 70.5% for SEC (n=370) (nominal p<0.001). These results were confirmed in the maintenance set, with 85.7% of the BZK q4w/q4w group (n=147) achieving PASI 90 versus 73.7% of the SEC group (n=354) (nominal p=0.002) and 86.5% of the BZK q4w/q8w group (n=215) achieving PASI 90 versus 73.7% of the SEC group (n=354) (nominal p<0.001).
The investigators also examined proportions of patients achieving clear or almost clear skin, as measured by the Investigator Global Assessment (IGA) scale, where zero is clear and one represents almost clear. A similar response pattern was observed in the difference between BZK and SEC at Weeks 16 and 48. At Week 16, 85.5% of the BKZ q4w group (n=373) achieved IGA 0/1 compared with 78.6% of the SEC group (n=370; nominal p=0.012). At Week 48, the proportions were 83.9% of the BKZ q4w or q4w/q8w group (n=373) and 73.8% of the SEC group (n=370) (nominal p<0.001). The findings were confirmed in the maintenance set, which showed that 87.1% of the BZK q4w/q4w group (n=147) achieved IGA 0/1 at Week 48 compared with 77.1% of the SEC group (n=354; nominal p=0.005). Similarly, 86.0% of the BZK q4w/q8w group (n=215) achieved IGA 0/1 at Week 48 versus 77.1% of the SEC group (n=354; nominal p=0.013).
Reich presented results for the secondary endpoint measuring speed of onset (PASI 75 at Week 4), noting that for this parameter, patients in the BZK group would have received just one injection at Week 0. A significant difference was found between treatments, with 71.0% of the BZK q4w group achieving PASI 75 versus 47.3% of the SEC group (p<0.001). Reich also showed that 35.9% of the BZK q4w group achieved PASI 90 at Week 4 compared with 17.6% of the SEC group (nominal p<0.001).
Regarding safety, rates of treatment-emergent adverse events (TEAEs) were as expected from previously published Phase III trials and there were no new safety signals. Incidences of serious infections, inflammatory bowel disease, suicidal ideation and behaviour, and death were all low for both BZK and SEC. Both drugs showed similar rates of any TEAE (321 [86.1%] patients on BKZ versus 301 [81.4%] patients on SEC) and serious TEAEs (22 [5.9%] patients on BKZ versus 21 [5.7%] patients on SEC). There was a higher rate of Candida infections in the BZK group, with 79 (21.2%) patients affected compared with 17 patients (4.6%) in the SEC group. This was mainly due to oral candidiasis, which occurred in 72 (19.3%) patients on BZK compared with 11 (3.0%) patients on SEC during Weeks 0-48 (all patients). In the BZK group the vast majority of the oral candidiasis cases (70/72; 97.2%) were mild or moderate; none were serious, and none led to treatment discontinuation.
Reich concluded that the faster and higher response levels with BZK compared to SEC indicate that optimal efficacy of treatment requires inhibition of both IL-17A and IL-17F in patients with moderate-to-severe plaque psoriasis.
Efficacy and Safety of Ixekizumab in Chinese Patients with Moderate-to-Severe Plaque Psoriasis: 12-Week Results from a Phase III Study
Xia Li
Results of another IL-17A inhibitor, IXE, were presented in an ePoster.6 The three Phase III UNCOVER trials previously demonstrated that IXE achieved rapid onset and high efficacy with a consistent safety profile in patients with moderate-to-severe plaque psoriasis.7,8 However, until now, the efficacy and safety of IXE in Chinese patients with psoriasis had not been reported. The current study compared the efficacy and safety of IXE with PBO in Chinese patients with moderate-to-severe plaque psoriasis.6
A total of 438 patients were randomised in a 2:2:1 ratio to receive IXE 80 mg q4w (n=174), IXE 80 mg every 2 weeks (q2w): n=176), or PBO (n=88). During the induction dosing period (Weeks 0–12) patients in the IXE groups received a 160 mg starting dose as two injections. The co-primary endpoints were sPGA 0/1 and PASI 75 at Week 12.
Regarding baseline characteristics, the average weight was 72 kg in the IXE q4w group, 74 kg in the IXE q2w group, and 73 kg in the PBO group. Mean PASI was 25 in the IXE q4w and PBO groups and 26 in the IXE q2w group. Previous use of biologic therapy was highest in the PBO group (12.5%), followed by the IXE q4w (9.2%) and IXE q8w (8.0%) groups.
The authors demonstrated that both IXE regimens achieved superior efficacy compared to PBO. At Week 12, the sPGA 0/1 response rates were 3.4%, 79.9%, and 86.4% in the PBO, IXE q4w, and IXE q2w groups, respectively (p≤0.001 for IXE groups versus PBO). The PASI 75 response rates were 8.0%, 87.4%, and 93.8% for the PBO, IXE q4w, and IXE q2w groups, respectively (p≤0.001 for IXE groups versus PBO).
Rapid onset of efficacy was noted by the authors, with both IXE q4w and IXE q2w groups achieving higher PASI 50 response rates compared to PBO (32.2%, 28.4%, versus 3.4%) as early as Week 1 and PASI 75 (24.1%, 21.0%, versus 0%), and sPGA 0/1 (21.3%, 17.6%, versus 0%) at Week 2.
Overall, during the 12 weeks 297 (67.8%) patients reported ≥1 TEAE and 6 (1.4%) patients reported a serious adverse event. Most TEAEs were mild-to-moderate. Looking at the three groups separately, TEAEs occurred in 47 (53.4%) patients in the PBO group, 123 (70.7%) patients in the IXE q4w group, and 127 (72.2%) patients in the IXE q2w group. The most common TEAE was upper respiratory infection, which occurred in 9 (10.2%), 25 (14.4%), and 28 (15.9%) patients in the PBO, IXE q4w, and IXE q2w groups, respectively. The second most common TEAE was injection site reaction, which occurred in no patients in the PBO group, 13 (7.5%) in the IXE q4w group, and 19 (10.8%) in the IXE q2w group. Pruritis affected 2 (2.3%), 8 (4.6%), and 8 (4.5%) patients in the PBO, IXE q4w, and IXE q2w groups, respectively, while corresponding rates of tinea pedis were 0 (0%), 9 (5.2%), and 9 (5.1%). There were no deaths.
The authors concluded that IXE demonstrated significant rapid onset and high efficacy with a favourable safety profile in Chinese patients with moderate-to-severe plaque psoriasis during the induction dosing period.
Maintenance of Response Through up to 5 Years of Continuous Guselkumab Treatment of Psoriasis: The Voyage 2 Phase III Trial
Kristian Reich
VOYAGE 2 was a Phase III, randomised, double-blind, PBO/active comparator-controlled trial that evaluated the efficacy and safety of the IL-23 inhibitor GUS in patients with moderate-to-severe psoriasis.9,10 The authors presented the results from up to 5 years of continuous GUS treatment in an ePoster.
Patients were randomised to three groups:
1. GUS 100 mg at Weeks 0/4/12, then q8w;
2. PBO at Weeks 0/4/12, GUS 100 mg at Weeks 16/20, then q8w; or
3. adalimumab (ADA) 80 mg at Week 0, 40 mg at Week 1, then 40 mg q2w until Week 23.
Randomised withdrawal occurred between Weeks 28–72 followed by open-label GUS 100 mg q8w from Weeks 76–252. Efficacy was evaluated until Week 252 and safety was evaluated until Week 264. Efficacy assessments included ≥90% or 100% improvement in PASI 90/100 and IGA 0/1 or 0.
Through Week 252, 74.4% of patients completed the study agent. Results were presented for the GUS group (which includes both patients randomised to GUS at baseline and patients randomised to PBO who crossed over to receive GUS at Week 16; n=657) and for the ADA/GUS group (patients randomised to ADA at baseline who crossed over to receive GUS at or after Week 28; n=210).
The authors showed that the proportions of patients achieving PASI 90, PASI 100, IGA 0/1, and IGA 0 were consistent from Weeks 100 to 252 in both GUS and ADA/GUS treatment groups. As an example, the proportions achieving PASI 90 at Weeks 100 and Weeks 252, respectively, were 79.1% and 82.0% in the GUS group and 81.4% and 79.1% in the ADA/GUS group (Figure 2).
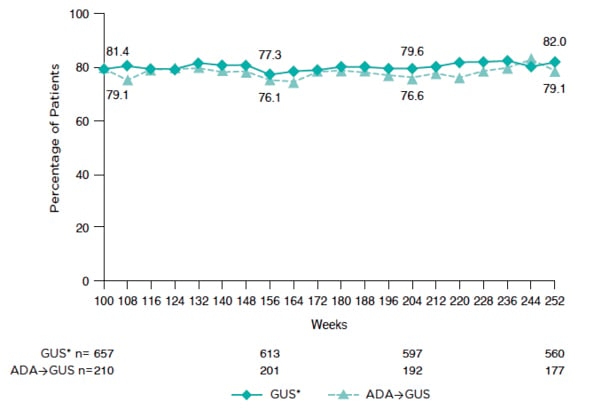
Figure 2: Proportion of patients who achieved PASI 90 response through Week 252.
*Includes patients randomised to GUS at baseline and to PBO who crossed-over to receive GUS at Week 16.
ADA: adalimumab; GUS: guselkumab; PASI: Psoriasis Area and Severity Index.
No new safety signals were identified up to Week 264. The authors concluded that high levels of efficacy were maintained from Week 100 to 252 with continuous GUS treatment across multiple endpoints in VOYAGE 2, and GUS treatment was well-tolerated.
Long-Term Efficacy of Tildrakizumab: A Pooled Analysis of ReSURFACE 1 and ReSURFACE 2 Through up to 4 Years of Treatment
J. Mark Jackson
TIL, an IL-23 inhibitor, was previously evaluated in patients with moderate-to-severe plaque psoriasis in 2 Phase III, randomised, controlled trials: reSURFACE 1 and reSURFACE 2.11,12,13 In an ePoster, the authors presented a post hoc analysis evaluating the 4-year (196 weeks) efficacy of TIL 100 mg based on the proportion of patients achieving favourable PASI and PGA scores in a pooled analysis of the two trials.
This analysis included data from patients continuously receiving TIL 100 mg administered at Weeks 0 and 4 and every 12 weeks (q12w) thereafter in the 3-part reSURFACE 1 (64 weeks) and reSURFACE 2 (52 weeks) trials and their optional open-label extension periods of 64–256 and 52–244 weeks, respectively. A total of 329 patients achieved PASI 75 at Week 28 and continued receiving TIL 100 mg into Part 3; of these, 302 patients entered the extension period and 253 (83.8%) were assessed at Week 196.
The authors reported that at Week 196, 85.4% of patients taking TIL 100 mg achieved PASI 75, 60.3% achieved PASI 90, and 32.8% achieved PASI 100 (Figure 3). The proportion of patients receiving TIL 100 mg with a PGA of 0/1 was 63.3% at Week 196.
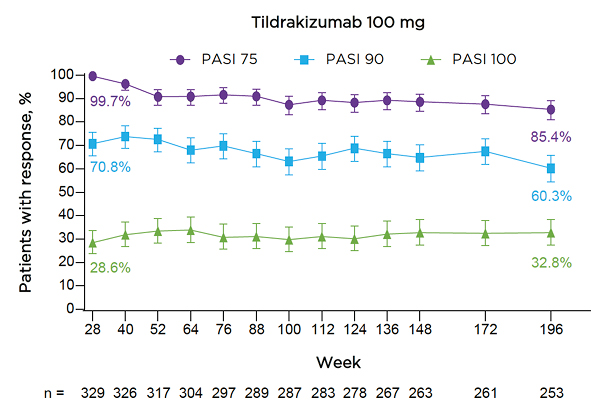
Figure 3: Proportions of patients receiving tildrakizumab 100 mg achieving PASI 75/90/100 responses through Week 196.
Error bars show the 95% confidence interval.
Numbers below the graph represent patients with observed data at each corresponding time point; missing data were handled using multiple imputation.
PASI: Psoriasis Area and Severity Index.
The authors concluded that TIL 100 mg provided durable efficacy through up to 196 weeks of treatment for moderate-to-severe plaque psoriasis.
Efficacy and Safety of Risankizumab Versus Methotrexate in Patients with Moderate-to-Severe Plaque Psoriasis: Results from an Ongoing Phase III Study in Brazil
Tania F. Cestari
This ePoster focused on the IL-23 inhibitor RZB in patients with moderate-to-severe plaque psoriasis in Brazil. The multicentre Phase III trial compared the safety and efficacy of RZB with MTX, which has been used to treat psoriasis for decades, but is associated with gastrointestinal, hepatic, renal, and haematopoietic abnormalities. 14,15
The study design included a 30-day screening period, followed by 28 weeks of double-blind treatment, and 84 weeks of open-label treatment. For the double-blind period, patients were randomised 1:1 to receive RZB 150 mg subcutaneously at Weeks 0, 4, and 16 and weekly MTX PBO capsules, or MTX 5 mg once weekly (with dose escalation to 25 mg based on clinical response and tolerability) and RZB PBO subcutaneously injections at Weeks 0, 4, and 16.
The primary efficacy endpoints (both at Week 28) were the proportions of patients who achieved ≥90% reduction from baseline in PASI 90 and sPGA of clear or almost clear (0/1).
A total of 98 patients were randomly allocated to the two treatments (RZB: 50; MTX: 48). The authors reported that patients in both treatment groups had similar baseline characteristics. One-half of the patients in each group had a BMI ≥30 kg/m2. Mean PASI was 20.0 in the RZB group and 22.1 in the MTX group.
Regarding the primary efficacy endpoints, a significantly greater proportion of patients treated with RZB compared to MTX achieved PASI 90 (84.0% versus 35.4%; p<0.001) and sPGA 0/1 (90.0% versus 64.6%; p<0.01) at Week 28. The authors noted that the proportions of patients achieving PASI 90 and sPGA 0/1 were significantly greater in the RZB compared to MTX group at all time points from Weeks 8 and 4, respectively.
Safety was analysed in all patients who were randomised at baseline and received ≥1 dose of study drug. The authors stated that no new safety findings were observed. TEAEs occurred in 38 (77.6%) and 40 (83.3%) patients in the RZB and MTX groups, respectively. Serious TEAEs were observed in two (4.1%) patients receiving RZB and three patients (6.3%) receiving MTX. The authors concluded that the results support the favourable benefit–risk profile observed in prior studies of RZB.
Efficacy and Safety of Mirikizumab in Psoriasis: 52-Week Results from the OASIS-1 Randomised Controlled Trial
Andrew Blauvelt
This ePoster highlighted the efficacy and safety of the IL-23 inhibitor MIR in patients with moderate-to-severe plaque psoriasis through 52 weeks in the Phase III OASIS-1 trial.16
A total of 530 patients were randomised in a 4:1 ratio to receive MIR 250 mg or PBO q4w through Week 12. The primary endpoints were superiority of MIR versus PBO in achieving sPGA 0/1 with ≥2-point improvement and ≥90% improvement in PASI 90 from baseline at Week 16. Patients entering the maintenance period (16–52 weeks) with PASI 90 were re-randomised 1:1:1 to MIR 250 mg q8w (n=91), MIR 125 mg q8w (n=90), or PBO q8w (91). Major secondary endpoints were evaluated at Week 52.
At baseline, average body weight was 86.5 kg in the PBO group and 84.7 kg in the MIR group. Mean PASI was 23.5 and 22.3 in the PBO and MIR groups, respectively. Prior biologic therapy had been used by 31 (29.0%) patients in the PBO group and 139 (32.9%) in the MIR group.
The authors reported that at Week 16, 69.3% of patients in the MIR group achieved sPGA 0/1 compared with 6.5% of the PBO group, while PASI 90 was achieved by 64.3% and 6.5% patients receiving MIR and PBO, respectively (p<0.05). Looking at Week 52 results, sPGA 0/1 was achieved by 17.6% patients in the PBO group compared with 82.4% in the MIR 250 mg (p<0.05) and 85.6% in the MIR 125 mg (p<0.05) groups. Similar results were found for PASI 90 at Week 52.
The overall rates of TEAEs were similar across treatment groups during the induction and maintenance periods. More infections occurred in the MIR group compared to PBO but there were no new safety signals.







