Meeting Summary
Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, both members of the IL-17 family of proinflammatory cytokines. Bimekizumab provides rapid and long-term response in patients with moderate-to-severe plaque psoriasis. At the European Academy of Dermatology and Venereology (EADV) 2023 Congress, three posters were presented reporting 3-year results from the Phase III/IIIb clinical trials of bimekizumab in plaque psoriasis.
The first poster focused on the subgroup of patients in a pooled analysis who achieved a 90% or 100% improvement from baseline Psoriasis Area and Severity Index (PASI 90/100) or Investigator’s Global Assessment (IGA) of 0 or 1 (IGA 0/1) at Week 16, and showed that these responses could be maintained through to 3 years of treatment. The second poster focused on another subgroup of patients in the pooled analysis, who had scalp, nail, or palmoplantar involvement at baseline, and reported the proportion of patients achieving clearance in these high-impact areas over 3 years. High levels of complete scalp and palmoplantar clearance were shown after 16 weeks, which were sustained through to Year 3. Levels of complete nail clearance increased through the end of Year 1, reflective of the longer time required for nail growth, and were then sustained to the end of Year 3. The third poster presented data from the BE READY randomised withdrawal trial. The analysis focused on patients achieving PASI 90 at Week 16, who were then re-randomised to placebo. Around one-third of these patients retained PASI 75 until Week 56. For the two-thirds of patients who dropped below this level, restarting bimekizumab 320 mg every 4 weeks as ‘escape’ treatment led the majority to return to PASI 90 after 12 weeks. Both groups of patients could enter the subsequent open-label extension, and high responses were sustained through 3 years, showing that treatment interruption did not meaningfully impact long-term disease control.
The results presented in these posters show that high levels of response can be achieved with bimekizumab through 3 years of treatment. Initial responses were well-maintained; patients with scalp, nail, or palmoplantar involvement showed clearance in these high-impact areas; and long-term response was not affected by withdrawal and re-treatment.
Introduction
Psoriasis symptoms can be not only uncomfortable, irritating, and painful, but can also impact several aspects of a patients’ daily life, such as work, social activities, and clothing choice, especially when they affect areas such as the scalp, palm, nails, and soles. Furthermore, depression, anxiety, stress, insomnia, and substance abuse have all been found to be higher in people with psoriasis compared with people without a skin condition.1-3 Factors important to patients when discussing treatment options include overall symptom relief, rapid symptom relief, achieving and maintaining clear skin, effectiveness in ‘high-impact’ areas (such as the nails, scalp, palms, and soles), and the occurrence of side effects.3 As psoriasis is a chronic disease, and loss of response is observed with some therapies over time, 4 studying long-term efficacy of new treatments is important. These findings highlight the need for long-term, safe, and effective treatments for control of psoriasis symptoms, that can lead to a better quality of life. Furthermore, patients with moderate-to-severe plaque psoriasis may report interruptions in biologic treatment. It is thus important to understand how long responses can be maintained after treatment withdrawal, and whether responses can be recaptured and maintained upon re-treatment.
Phase III/IIIb Trials of Bimekizumab in Moderate-to-Severe Plaque Psoriasis
Psoriasis is an autoimmune disease that occurs due to aberrant interactions between epidermal keratinocytes and immune system cells. Such interactions include overexpression by a number of innate and adaptive immune system cells, most prominently T helper 17 lymphocytes, of the pro-inflammatory cytokines IL-17A and IL-17F. These cytokines can stimulate production of inflammatory mediators and subsequent keratinocyte proliferation, leading to the thick, scaly, erythematous plaques indicative of psoriasis.5-7
As the expression patterns of these cytokines differ,8 targeting both in people with psoriasis may be advantageous. Indeed, dual and selective inhibition of IL-17A and IL-17F by the monoclonal IgG1 antibody bimekizumab9 may lead to more complete suppression of inflammatory responses associated with psoriasis than inhibition of IL-17A alone.10 Bimekizumab is typically administered subcutaneously to adults with plaque psoriasis at a dose of 320 mg, given every 4 weeks for the first 16 weeks, then every 8 weeks.11
In Phase III/IIIb clinical trials in patients with moderate-to-severe plaque psoriasis, bimekizumab use led to rapid and superior efficacy compared with placebo12,13 and with the monoclonal antibodies ustekinumab,12 adalimumab,14 and secukinumab.15 Studies have also shown long-term durability of bimekizumab response. The most common treatment-emergent adverse events in patients treated with bimekizumab were nasopharyngitis, oral candidiasis, and upper respiratory tract infection. These were predominantly mild or moderate. Discontinuation due to adverse events was Iow.12-16
These clinical trials include three large Phase III studies: BE VIVID,12 BE READY,13 and BE SURE,14 and one Phase IIIb study: BE RADIANT.15 For the bimekizumab arms, 320 mg was administered Q4W until Week 16 in all trials. At this point in each study, except BE VIVID, a subset of the Q4W group were switched to 8-weekly dosing (Q8W) throughout the maintenance period. Maintenance period end from baseline was 48 weeks from baseline in BE RADIANT, 52 weeks in BE VIVID and BE SURE, and 56 weeks in BE READY.12-15
At the end of BE VIVID, BE READY, and BE SURE, eligible patients receiving bimekizumab could enter an OLE trial named BE BRIGHT.16 BE RADIANT had an OLE period included.15 Patients in the Phase III studies received treatment for up to 4 years (to OLE Week 144), and those in BE RADIANT for up to 3 years (Week 144/OLE Week 96).15,16 For the OLE stages, all patients receiving bimekizumab with PASI <90 at the end of the maintenance period were initially administered a BKZ Q4W regimen. Patients achieving PASI ≥90 at the beginning of the OLE period who were on a BKZ Q4W regimen at the end of the first year were randomised 1:1 in BE RADIANT and 4:1 in BE BRIGHT to Q4W or Q8W.15 Patients achieving PASI ≥90 at the beginning of the OLE period who were on a BKZ Q8W regimen at the end of the first year remained on this dose.15,16 In BE BRIGHT, at OLE Week 24, patients achieving PASI ≥90 could be switched to Q8W by the investigator, with the remainders re-assigned to BKZ Q8W from OLE Week 48 or later.16 In BE RADIANT, all patients were switched to BKZ Q8W from OLE Week 16 (Week 64) or later.15
Throughout these trials, the main assessment tool was the PASI, which ranks degree of severity and percentage of surface involved for a total PASI score of 0−72.17 In these trials, response was defined as percentage reduction in score, e.g., PASI 90 denotes a ≥90% reduction in PASI score. Also used in these studies was the Investigator’s Global Assessment (IGA), which rates psoriasis from 0 (clear) to 4 (severe psoriasis). Response was defined as achieving a score of 0 or 1 (almost clear), with a two-point or bigger improvement from baseline.12-16
Long-Term Response Maintenance in Bimekizumab Week 16 Responders
Diamant Thaçi
Response to some psoriasis therapies may wane over time.4 As such, it is important to examine the long-term effects of a psoriasis treatment. A poster presented at the EADV Congress 2023 by Diamant Thaçi, Institute and Comprehensive Center for Inflammation Medicine, University of Lübeck, Germany, included pooled data from the 1,362 participants in the bimekizumab Phase III/IIIb trials who were initially randomised to BKZ Q4W. Data were reported for the combined dosing groups (BKZ Total) and for patients who received a BKZ Q4W/Q8W/Q8W (initial/maintenance/OLE) regimen,18 which is consistent with the approved dosing regimen of bimekizumab for psoriasis.11,19 Maintenance of PASI 90, PASI 100, and IGA 0/1 responses from baseline through Year 3 (Week 144/OLE Week 96) were reported in Week 16 PASI 90, PASI 100, and IGA 0/1 responders, respectively. Data were reported using modified non-responder imputation,20 where patients who discontinued due to lack of efficacy or treatment-related adverse events were considered non-responders at subsequent timepoints, with other missing data imputed using multiple imputation methodology.18
Baseline characteristics of all pooled participants who achieved PASI 90 (n=995), PASI 100 (n=719), or IGA 0/1 (n=985) at Week 16, and later entered the OLE phase were similar. Mean ages were approximately 45 years, about 70% were male, 88% were White, and mean psoriasis duration was around 18.2 years. Similarly, approximately 78% in each group received prior systemic therapy, and around 39% received prior biologic therapy. Baseline PASI mean scores were all about 21, with percentages for IGA 3/4 around 66/34%.18
As can be seen in Figure 1, of the 1,362 patients randomised to receive BKZ Q4W over the initial treatment period, at Week 16 86.9% achieved PASI 90, 62.4% achieved PASI 100, and 86.9% achieved IGA 0/1.18 For the BKZ Total group, analysis at Week 48, the last common timepoint for assessment across the trials before OLE entry, found that 96.0%, 88.7%, and 95.8% of Week 16 PASI 90, PASI 100, and IGA 0/1 responders, respectively, maintained their responses.
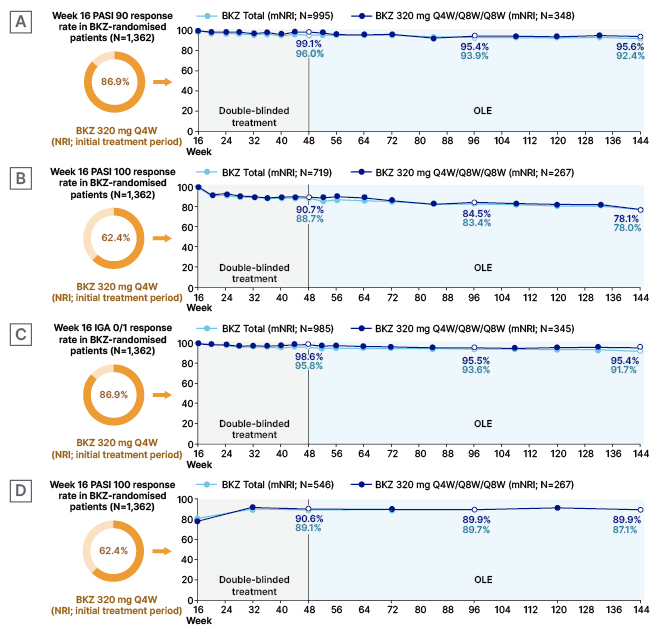
Figure 1: Maintenance in Week 16 bimekizumab-treated responders who entered the BE BRIGHT or BE RADIANT open-label extensions (modified non-responder imputation) of PASI 90 in Week 16 PASI 90 responders (A), PASI 100 in Week 16 PASI 100 responders (B), IGA 0/1 in Week 16 IGA/01 responders (C), and DLQI 0/1 in Week 16 PASI 100 responders (D).18
Week 16 responses are reported for all BKZ-randomised patients; response rates from Week 16 up to Week 144 are reported among patients randomised to BKZ at the start of BE SURE, BE READY, BE VIVID, and BE RADIANT achieved a PASI 90, PASI 100, or IGA 0/1 response at Week 16, and entered the relevant OLE. To pool data across all four studies, Week 52 and 56 data from the feeder studies were not included; timepoints after Week 48 are from the BE BRIGHT/BE RADIANT OLEs. DLQI responses are at visits
common to BE SURE, BE READY, and BE RADIANT. Data were reported using mNRI, where patients who discontinued due to lack of efficacy or treatment-related adverse events were considered non-responders at subsequent timepoints.
BKZ: bimekizumab; DLQI: Dermatology Life Quality Index; IGA: Investigator’s Global Assessment; mNRI: modified non-responder imputation; NRI: non-responder imputation; OLE: open-label extension; PASI: Psoriasis Area and Severity Index; PASI 90/100: ≥90%/100% improvement from baseline in Psoriasis Area and Severity Index; Q4W: every 4 weeks; Q8W: every 8 weeks.
During the OLE period, marginal decreases were seen in the proportion of patients who maintained PASI 90 and IGA 0/1, with >90% of the BKZ Total group and >95% of patients who received BKZ Q4W/Q8W/Q8W maintaining PASI 90 and/or IGA 0/1 to Week 144. PASI 100 in Week 16 PASI 100 responders was maintained in 78.0% of patients at Week 144. Across all timepoints, level of response was similar in patients who received the BKZ Q4W/Q8W/Q8W dosing regimen compared to the BKZ Total group (Figure 1).18
Also reported in this poster were Dermatology Life Quality Index (DLQI)21 0/1 responses in Week 16 PASI 100 responders. The DLQI is a 10-item questionnaire where patients rate how psoriasis has affected several domains over the last week, including skin symptoms; treatment efficacy; and social, work, and relationship activities. DLQI score ranges from 0−30, with a score of 0/1 denoting ‘no effect on a patient’s life’.12-16,21,22 For the Week 16 PASI 100 responders, DLQI mean±standard deviation (SD) baseline score was 10.7±6.4, representing a ‘moderate’ to ‘very large’ effect on a patient’s life.22 Figure 1D shows that among Week 16 PASI 100 responders, 62.4% reported DLQI 0/1 scores at this timepoint. This rose to 89.1% by Week 48, a level maintained through Week 144. Response rates were very similar regardless of dosing regimen.18
Also reported in this poster was that in this Week 16 responder cohort, study discontinuation due to loss of efficacy and adverse events during the maintenance period was 8 out of 1,102 (0.7%) and during the OLE period was 77 out of 1,102 (7.0%).
The authors concluded that “pooled data from five trials found that, among Week 16 responders, high clinical responses were maintained through 3 years of bimekizumab 320 mg treatment.”18
Long-Term Bimekizumab Efficacy in High-Impact Areas
Joseph F. Merola
‘High-impact’ areas for people with psoriasis include the scalp, hands (including the palms and nails), and the soles of the feet.3 Psoriatic lesions in these areas are associated with both treatment challenges and reduced health-related quality of life.23 Building on a previous presentation of 2-year bimekizumab efficacy in these ‘high-impact’ areas,24 Joseph F. Merola, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts, USA, presented a poster that reported responses in high-impact areas over 3 years (144 weeks) for bimekizumab-randomised patients pooled across all the Phase III/IIIb trials described above.25
For this analysis, scalp IGA and palmoplantar IGA (pp-IGA) were assessed and rated from 0 (clear) to 4 (severe). For nails, the modified Nail Psoriasis Severity Index (mNAPSI) was used, where each nail was scored 0–13 (maximum total score for hands: 130) in regard to onycholysis/oil drop dyschromia, nail plate crumbling, pitting, splinter haemorrhages, leukonychia, red spots in lunula, and nail bed hyperkeratosis. Patients with baseline scalp IGA or pp-IGA ≥3 or mNAPSI score >10 were included in the high-impact areas analysis. Proportions of patients who achieved complete regional clearance (0 scores) are reported through Year 3 (study Week 144/OLE Week 96). As above, data are reported using modified non-responder imputation.25
Baseline characteristics were similar for patients with scalp IGA ≥3 (n=821), pp-IGA ≥3 (n=193), and mNAPSI >10 (n=377), regarding mean age (around 45 years) and race (around 85% White), but differed in regard to gender (69.3%, 74.6%, and 83.8% male, respectively). The groups had a similar duration of psoriasis (around 18 years) and any prior biologic therapy (around 37%), but differed slightly in regard to receiving any prior systemic therapy (77.3%, 84.5%, and 78.8%, respectively).25
Mean±SD baseline PASI scores were all around 22 for patients with scalp IGA ≥3, pp-IGA ≥3, and mNAPSI >10, with mean DLQI scores around 11. Respective weight of respective IGA 3/4 scores were slightly different, being 64.2/35.8%, 56.5/43.0%, and 56.2/43.2%. Baseline scores were also provided in regard to each score across each category. While mean±SD scalp IGA scores for patients with scalp IGA ≥3, pp-IGA ≥3, and mNAPSI >10 were similar (around 3.0), respective mean±SD mNAPSI scores were slightly different, at 11.6±17.8, 21.9±28.0, and 31.0±20.5, as were respective mean pp-IGA scores: 0.9±1.3, 3.2±0.4, and 1.3±1.4.25
In this poster, scores were reported at Weeks 48, 96, and 144. As Figure 2 shows, for scalp IGA and pp-IGA, complete clearance was high at Week 16. At Week 48, respective percentages of complete clearance were 88.0% and 90.6%. Similar percentages, of 83.7% and 91.6%, respectively, were shown at Week 144. Reflective of nail growth time, at Week 16, around 20–30% of people with baseline mNAPSI >10 achieved clearance at this timepoint. By Week 48, nail clearance was seen in 64.4% of patients, increasing to 69.5% by Week 144. For all assessments, response to bimekizumab in high impact areas was similar across the 3 years, regardless of dosing regimen (Figure 2).25
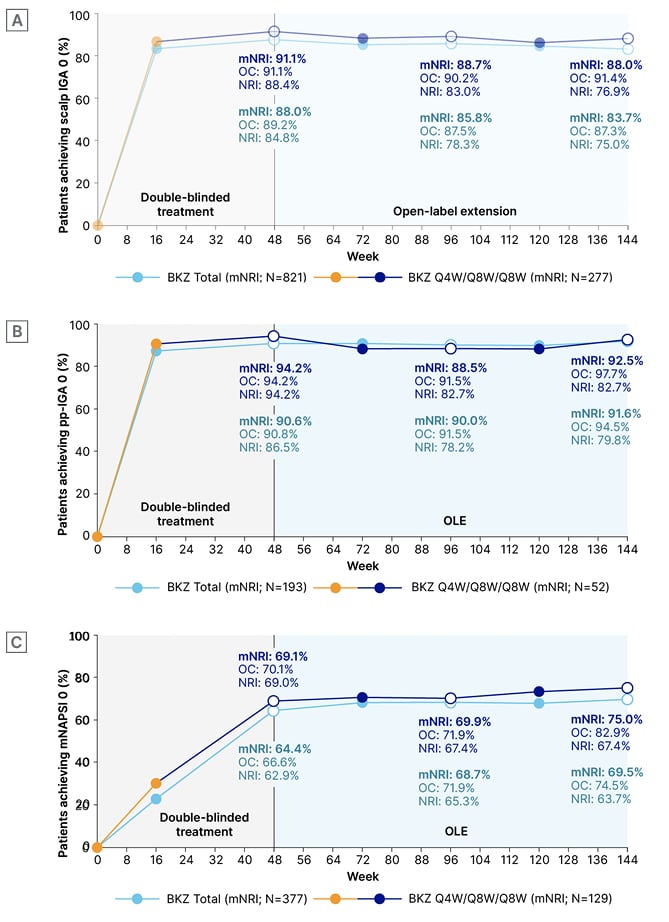
Figure 2: Complete clearance over 3 years (modified non-responder imputation, non-responder imputation, and observed case) of scalp psoriasis (scalp IGA 0) in patients with baseline scalp IGA ≥3 (A), palmoplantar psoriasis (pp-IGA 0) in patients with baseline pp-IGA ≥3 (B), and nail psoriasis (mNAPSI 0) in patients with baseline mNAPSI >10 (C).24
BKZ Total patients received BKZ 320 mg Q4W to Week 16, then either Q4W or Q8W in the maintenance period and OLE. BKZ Q4W/Q8W/Q8W patients received BKZ 320 mg Q4W to Week 16, then BKZ Q8W throughout the maintenance period, and on OLE entry. As no scalp, palmoplantar, or nail outcomes were collected at Week 48 in BE VIVID, Week 52 data were included at the Week 48 timepoint. To pool data across all four studies, Week 52/56 data from the feeder studies were not included; timepoints after Week 48 are from the BE BRIGHT/BE RADIANT OLEs. For mNRI analyses, patients who discontinued due to lack of efficacy or treatment-related adverse events were considered non-responders at subsequent timepoints.
BKZ: bimekizumab; IGA: Investigator’s Global Assessment; mNAPSI: modified Nail Psoriasis Severity Index; mNRI: modified non-responder imputation; NRI: non-responder imputation; OC: observed case; OLE: open-label extension; pp: palmoplantar; Q4W: every 4 weeks; Q8W: every 8 weeks.
The authors concluded that “over 3 years, high percentages of patients treated with bimekizumab achieved complete clearance of scalp, palmoplantar, and nail psoriasis, regardless of dosing regimen.”25
Bimekizumab Response Through 3 Years in Patients Who Stopped and Then Restarted Treatment
Antonio Costanzo
For a variety of reasons, patients treated with biologics may have therapy interruptions. Another poster presented at the EADV Congress 2023, by Antonio Costanzo, Dermatology, Humanitas Clinical and Research Centre, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), Rozzano, Milan, Italy, reported data from patients who received bimekizumab in the initial treatment period in the BE READY trial, then continued into BE BRIGHT.13,16,26 In BE READY, patients who were Week 16 PASI 90 responders were re-randomised at Week 16 to receive maintenance treatment with BKZ Q4W or Q8W (not reported here), or to placebo (n=105) over Weeks 16–56. Previous analysis of the placebo cohort found that the median time to loss of PASI 90 was 28 weeks from last bimekizumab dose,27 with median time to loss of PASI 75 (considered to be a relapse) being 32 weeks.13
This poster reported long-term outcomes in patients from the withdrawal cohort who received bimekizumab in the BE BRIGHT OLE. A total of 33 patients maintained a response at PASI ≥75 throughout the withdrawal period (the Weeks 16–56 placebo group), and only resumed treatment with BKZ Q4W from Week 56. A total of 66 patients relapsed to PASI <75 during the withdrawal period, and were entered into a 12 week escape arm (BKZ Q4W) before entering the OLE. These patients either received BKZ Q4W (n=54) or Q8W (n=12) on entering the OLE according to PASI response, as described above. At Week 80, those in Q4W achieving PASI 90 were switched to Q8W, at the investigator’s discretion, with all switched from Week 104 or later.26
The 66 patients in the Escape group were slightly older (mean±SD: 43.2±11.8 years) than the 33 patients who remained in the placebo group over Weeks 16–56 (n=33; 41.2±10.7 years), slightly more were male (75.8% versus 66.7%), and a slightly lower percentage were White (89.4% versus 97.0%). Disease duration was longer in the group of patients who relapsed and entered the escape arm versus the patients who maintained PASI 75 (mean±SD: 20.6±13.0 versus 14.6±8.3 years), PASI was slightly higher (19.7±7.5 versus 18.2±4.8), and percentages of IGA 3/4 were slightly different (68.2/31.8% versus 72.7/27.3%). Much higher percentages of the Escape group had received any prior systemic therapy (81.8% versus 57.6%) or prior biologic therapy (50.0% versus 18.2%), compared with the placebo group.26
As shown in Figure 3, for the Escape group, 59 out of 65 (90.8%) regained PASI 90, and 41 out of 65 (63.1%) achieved PASI 100 at Escape Week 12 (OLE Week 0) following bimekizumab re-treatment (one patient missed the Escape Week 12 visit). By OLE Week 96, PASI 90 was achieved in 55 out of 59 (93.2%) patients, and PASI 100 in 46 out of 59 (78.0%) patients. Of the patients who continued in the placebo arm until Week 56, 17 out of 33 (51.5%) maintained PASI 90, and 11 out of 33 (33.3%) achieved PASI 100. Results for this placebo group greatly improved following re-treatment with bimekizumab, up to OLE Week 96, with 27 out of 28 (96.4%) achieving PASI 90, and 24 out of 28 (85.7%) achieving PASI 100.26
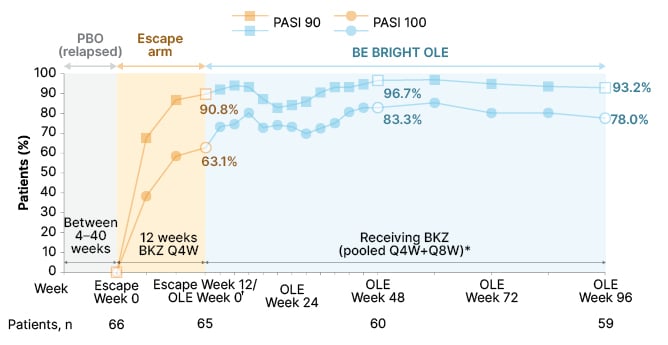
Figure 3: Achievement of PASI 90 and PASI 100 over 3 years in patients re-treated with bimekizumab in the Escape group (observed case).25
*Data reported from BE BRIGHT OLE are pooled for patients who received BKZ 320 mg Q4W and Q8W; †patients in the Escape group had their OLE Week 0 study assessments at the end of the 12-week escape arm, having achieved PASI 50 at the end of the 12 weeks; 65 out of 66 patients had a PASI measurement recorded at Escape Week 12/OLE Week 0, as one patient missed this visit.
BKZ: bimekizumab; OC: observed case; OLE: open-label extension; PASI: Psoriasis Area and Severity Index; PASI 90/100: ≥90%/100% improvement from baseline in PASI; PBO: placebo; Q4W: every 4 weeks; Q8W: every 8 weeks.
These results, the authors concluded, “indicated that stopping bimekizumab for up to 40 weeks and restarting did not meaningfully impact long-term disease control.”26
Conclusion
These studies show that high levels of response can be achieved with bimekizumab through 3 years of treatment. Initial responses were well-maintained; patients with scalp, nail, or palmoplantar involvement showed clearance in these high-impact areas, and long-term response was not affected by treatment interruption.






