Meeting Summary
In this continuing medical education session at the 2024 Fall Clinical Dermatology Conference, James Q Del Rosso, Adjunct Clinical Professor of Dermatology at Touro University Nevada, Henderson, USA, and Karan Lal, Director of Pediatric Dermatology at Affiliated Dermatology, Scottsdale, USA, reviewed recent additions to the therapeutic armamentarium for acne and considered how to integrate new topical therapies into clinical practice in order to optimize outcomes for patients. Notable recent additions to dermatologists’ acne toolkit include newer dual and triple combination therapies, as well as agents with novel mechanisms, such as the androgen-receptor inhibitor clascoterone. Given the wide variety of topical therapies now available, Lal emphasized the importance of joint decision-making and individualized acne management that meets patients’ needs, as well as targeting underlying disease pathophysiology.
Patient-Centered Care
The seminar began by highlighting the importance of patient-centered and collaborative care that lays the foundation for successful acne treatment. Both faculty presenters stressed that, in addition to performing acne diagnosis assessment, healthcare professionals are encouraged to establish a connection with patients themselves in order to determine the optimal treatment approach. Other important steps in acne evaluation include a thorough review of patients’ medical history, medications (considering the potential for drug-induced acne), and past acne treatment.1,2 A focus on patient education and expectation management is also essential.
The Key Role of Topical Therapies
Del Rosso explained that different acne medications work at different points in the lifecycle of an individual type of acne lesion, with oral isotretinoin currently the only monotherapy option able to target all underlying pathogenic pathways and lesion types (Figure 1). Individual pilosebaceous follicles involved in the emergence of a given acne lesion develop independently of one another, meaning that many affected follicles are at different lifecycle stages at any given point in time. This accounts for why treatment for acne requires a reasonable duration of therapy over at least a few months to assess the initial therapeutic response. Diffuse thin layer application, and not only “spot” treatment to the acne-affected region, is necessary, as topical therapy treats both currently visible lesions but, most importantly, also works to reduce the emergence of newly developing acne lesions in other pilosebaceous follicles.
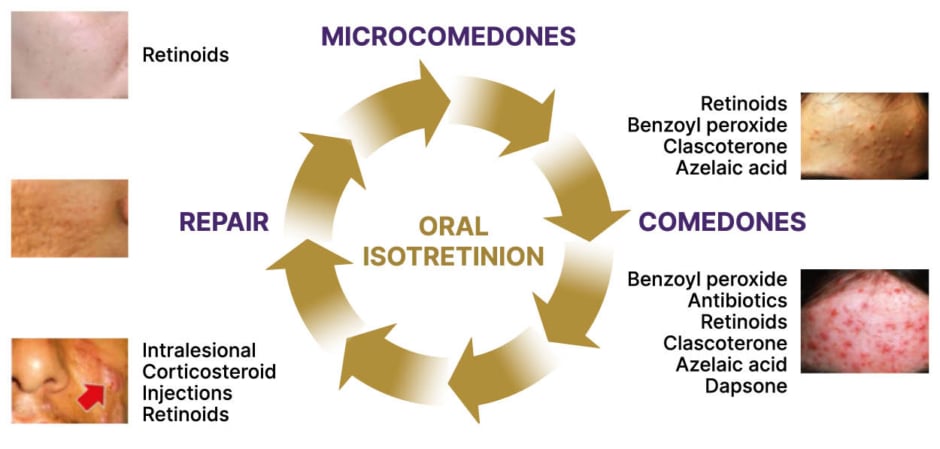
Figure 1: Correlation of acne lesion types and therapy selection.
This was created by James Q Del Rosso and used here with his permission.
Of the available treatment options, Del Rosso described topical therapies as the “foundation” of current acne management. This is demonstrated in the recently published American Academy of Dermatology (AAD) acne guidelines, which recommend using topical therapies that combine multiple mechanisms of action, limiting systemic antibiotic use as much as possible, and combining use of antibiotics with benzoyl peroxide to reduce the emergence of antibiotic-resistant bacteria at skin application sites.3
Del Rosso stressed that the appropriate use of these available topical therapies, in conjunction with proper skin care, is vital to achieving positive outcomes. In particular, it is important to emphasize to patients the importance of applying treatment to the entire face, and not just “spot treating” individual acne lesions, he noted, as described above.
Targeting Androgens
The four established pillars of acne pathogenesis are sebum production, follicular hyperkeratinization, Cutibacterium acne (C. acnes) colonization and proliferation of pro-inflammatory strains, and cascades of inflammation.4 Yet, as Del Rosso explained, the pivotal role that androgen hormones play in acne pathogenesis has previously been overlooked. We now know that the skin itself is an endocrine organ, capable of producing androgens, and that these hormones are implicated early on in the clinical cascade of acne pathogenesis.5 Notably, androgens stimulate sebum production by sebocytes within sebaceous glands in the skin, which creates a highly favorable microenvironment for growth of C. acnes bacteria within the pilosebaceous follicle.6 “If there are no androgens, there’s no sebum, and if there’s no sebum, there’s no acne; and that’s practically universally true,” Del Rosso remarked. Androgens also stimulate cytokines and other inflammatory pathways, leading to downstream effects that further promote lesion development (Figure 2).5
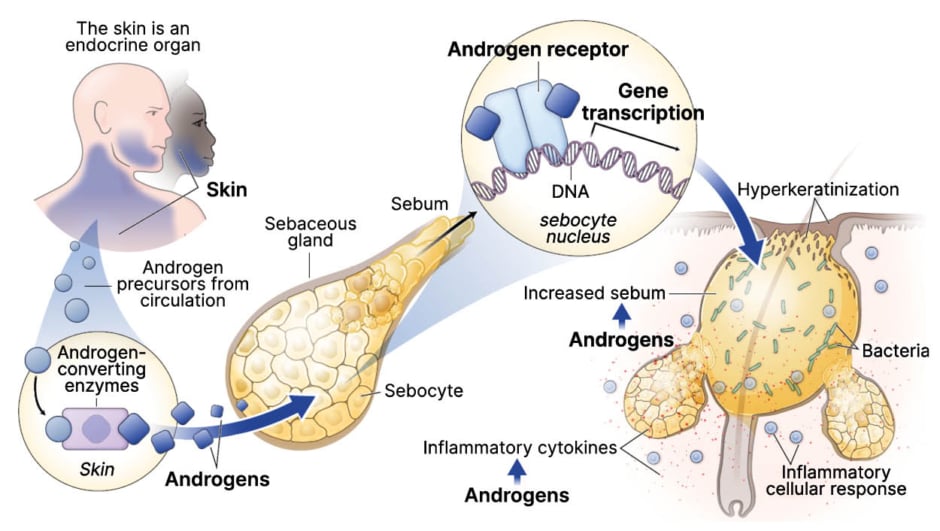
Figure 2: Acne vulgaris pathogenesis clinical cascade.
This was created by James Q Del Rosso and used here with his permission.
The skin has, therefore, emerged as a primary target for androgen inhibition and sebum modulation in acne management.5,6 Clascoterone 1% cream is a novel topical androgen receptor inhibitor approved for the treatment of acne vulgaris in patients 12 years of age and older.7 It directly targets and inhibits androgens in the skin by competing with androgens, primarily dihydrotestosterone (DHT), for binding to the androgen receptor within the cytoplasm of sebocytes, thereby inhibiting sebum production and reducing inflammatory cytokines.5-7 Clascoterone demonstrated favorable efficacy and safety in two randomized, vehicle-controlled, Phase III trials involving 1,440 male and female acne patients.8 At week 12, treatment success rates in the two trials ranged from 18.4–20.3% with clascoterone, and were significantly higher than with vehicle (6.5–9.0%).8 Treatment success was defined as an Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) and a ≥2-grade improvement from baseline and absolute change from baseline in non-inflammatory and inflammatory lesion counts. Del Rosso postulated that the cadence of this clinical response, and the onset of visible treatment effects, may be accelerated if topical androgen receptor inhibition with clascoterone is used as part of topical combination therapy, which is the usual standard of care with topical therapy for acne. Data from a 1-year, open-label extension study also support the longer-term use of clascoterone, with the potential to treat acne lesions on the trunk as well as the face.9 In this study, the longer patients were treated with topical clascoterone for acne, the better their clinical response in achieving clear or almost clear (IGA 0 or 1 rating) on both the face and on the trunk, Del Rosso pointed out (Figure 3).
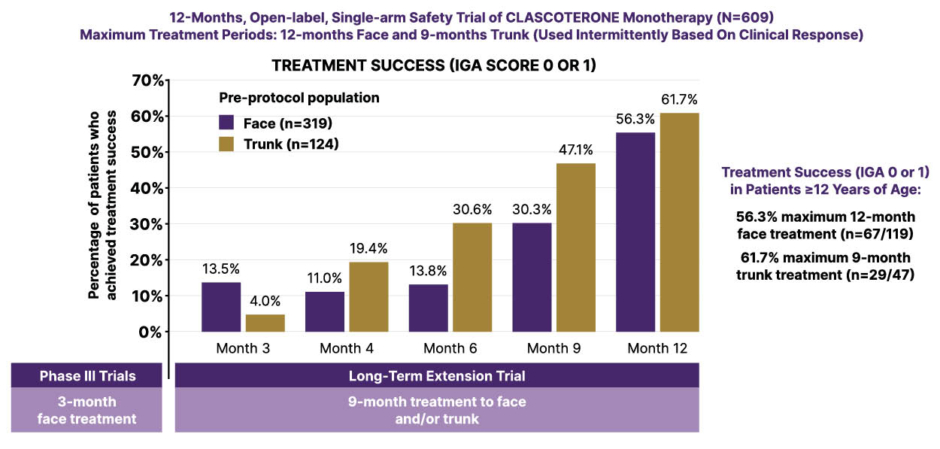
Figure 3: Long-term safety and efficacy of clascoterone cream 1%.10
Figure has been adapted from Eichenfeld L et al.10 2023
IGA: Investigator’s Global Assessment score.
Regarding the potential combination of clascoterone with other topical agents, Del Rosso emphasized tolerability and skin barrier effects as key considerations, alongside efficacy. Importantly, clascoterone 1% cream applied twice daily was shown not to impair the epidermal permeability barrier in a split-face study involving 49 patients. In this study, no significant increases in transepidermal water loss (TEWL) were seen, and clascoterone actually increased corneometry readings, which measure the magnitude of skin moisture levels, by 13% after 2 weeks of application, indicating an improvement in skin hydration.11
Combination Regimens
Combining treatments with different mechanisms can help to address the four major pillars of acne pathogenesis, and the introduction of clascoterone means we can now also target and inhibit androgens in the skin with topical therapy, Del Rosso remarked. Beyond the use of topical monotherapy agents that can be combined in a multistep regimen, a number of dual combination topical products are already well-established for the treatment of acne. These have the advantage of reducing the overall treatment burden for patients and include benzoyl peroxide (BPO) plus adapalene, BPO plus antibiotic (clindamycin or erythromycin), and clindamycin plus tretinoin.12 A novel combination formulation of BPO (3%) with tretinoin (0.1%) has also been developed where the individual agents are microencapsulated to resolve the problem of degradation of tretinoin by BPO.13
In a further therapeutic advance in the topical space, Del Rosso highlighted clindamycin 1.2%/adapalene 0.15%/BPO 3.1% gel as the first fixed-dose triple combination approach, combining an antibiotic, a non-antibiotic antibacterial agent, and a retinoid.14 Clindamycin is employed as the antibiotic in this combination, rather than erythromycin, due to its consistent acne lesion reductions as monotherapy and sustained efficacy over time.15 Del Rosso explained that the product itself is formulated with polymeric emulsion technology that partitions different active ingredients so they are delivered effectively while also augmenting skin tolerability.16,17 In two randomized, Phase III trials, patients experienced a ~50% reduction in inflammatory and non-inflammatory lesions after 12 weeks of treatment with the triple combination formulation.14 Additional post-hoc analysis showed this triple combination produces significantly greater improvement in acne lesion counts than the corresponding dyads, and that these reductions in lesions occur as early as Week 4, and are sustained over the course of the study.18
Addressing concerns around potential exposure to benzene, a known carcinogen, from BPO-containing acne products such as the new triple fixed-dose combination, Del Rosso acknowledged that many unanswered questions remain.19 A recent Citizen Petition by the independent analytical laboratory Valisure reported that BPO products have the potential to contain various quantities of benzene due to degradation of BPO, especially if transported and/or stored at above-ambient temperatures.20 This issue has been the subject of significant controversy regarding the actual risks related to these findings. In response, the American Acne and Rosacea Society (AARS) recommended that, until there is further guidance from the FDA, which is responsible for regulating and verifying the safety and stability of BPO products, patients should work directly with their dermatologist or healthcare provider to determine what course of action is most applicable to their case and comfort level. Del Rosso noted that the AARS has recommended some practical safety precautions, such as ensuring BPO-containing products are kept cool (storing in a refrigerator if possible), adhering to product expiration dates, and replacing BPO products every 3 months or less based on individual product expiration date requirements.
Clinical Practice Perspectives
In the final session of the seminar, Lal offered an insight into real-world acne management using two case studies from his own clinical practice. He reiterated the importance of a patient-centered approach, considering compliance (how many products a patient is willing to use), tolerability, and the severity of acne lesions, as well as patients’ motivation to treat. Choice of vehicle is also important, noted Lal, because this directly influences drug penetration, especially with fixed-dose combination products.21
The first case was a 16-year-old male with a 2-year history of acne who was initially prescribed BPO plus adapalene but showed poor compliance. Clinical examination revealed comedonal acne with a minimal inflammatory component and the decision was made to treat with the triple combination product. This was prescribed alongside a gentle skin cleanser to reduce irritation and a sunscreen-infused moisturizer (SPF 30+) to reduce the risk of photosensitivity from BPO. Best practices for safety and storage of the product were also discussed.
Case 2 was a 45-year-old female on bioidentical hormone replacement therapy with symptoms of perimenopause and longstanding acne on the lower face and jawline. Lal explained how this patient typifies “forgotten” patients with acne in middle age and pointed to evidence showing a link between compounded hormonal therapy (CHT) and increased acne incidence versus traditional HRT.22 Unfortunately, CHT prescriptions are not routinely tracked in the USA, but estimates suggest ~14% or more women may be on CHT, and that use is growing, making this a key question for dermatologists to ask their female patients, he noted.23 Lal explained that clascoterone was selected for this patient because it is a topical androgen hormone blocker with good tolerability, and good treatment responses have been attained thus far, including in this patient.
Conclusion
In conclusion, Del Rosso reiterated that acne management warrants individualized therapy, which also integrates skin care and other patient-specific factors. Comprehensive topical therapy, which is able to address the four major pathophysiologic pillars of acne, is now available, and optimal selection of therapy may also be correlated with acne lesion types. Notable treatment advances and recent additions to the topical acne toolkit include the novel androgen receptor inhibitor clascoterone and the first fixed-dose triple combination product. Ultimately, patients and dermatologists should reach a joint decision about acne treatment after discussion of the pros and cons of available therapies, he concluded.






