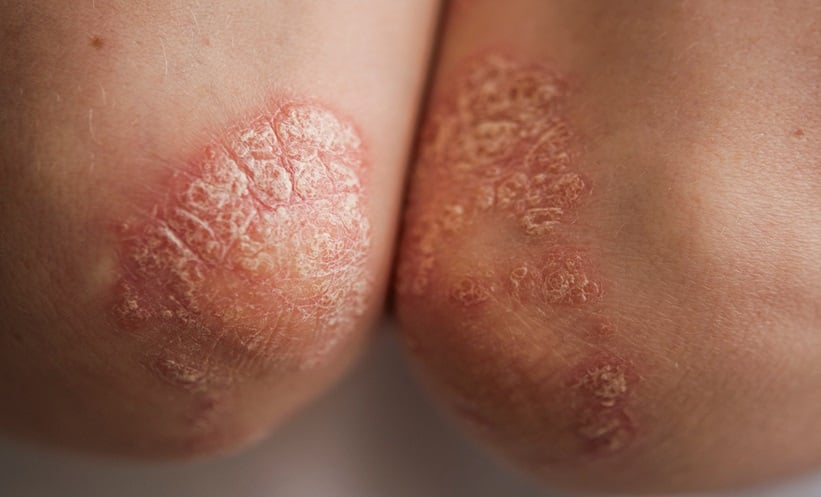
PSORIASIS, a chronic inflammatory disease, affects approximately 8 million adults in the United States and 2% of the global population. This condition causes significant physical discomfort, psychological distress, and economic burden, heavily impacting patients’ quality of life. Evidence-based treat-to-target approaches, effective in managing chronic diseases like diabetes and hypertension, are gaining traction in psoriasis management. These strategies aim to achieve and maintain low disease activity or remission using personalised treatment plans.
The introduction of biologics and targeted systemic therapies has enhanced treat-to-target strategies for psoriasis. The National Psoriasis Foundation (NPF) recommends reducing the total body surface area affected to 1% or lower within three months of treatment. Similarly, the European Academy of Dermatology and Venereology (EADV) guidelines set a goal of a 75% reduction in the Psoriasis Area and Severity Index (PASI75) within three to four months. However, many patients fail to meet these targets with topical treatments alone due to their limitations and adverse effects.
Tapinarof cream 1%, a novel nonsteroidal aryl hydrocarbon receptor agonist, has emerged as a promising solution for plaque psoriasis. In the PSOARING 1 and PSOARING 2 phase 3 trials, tapinarof demonstrated significantly higher PASI75 responses compared to placebo (36% and 48% versus 10% and 7%, respectively). Furthermore, it achieved complete disease clearance (PGA score of 0) in over 40% of patients in the PSOARING 3 long-term extension trial.
Beyond efficacy, tapinarof cream 1% offers flexibility, with no restrictions on application site, duration, or extent of use. The treatment provides durable responses, maintaining disease control for approximately four months off therapy, and exhibits no evidence of tachyphylaxis.
These findings underscore the potential of tapinarof cream 1% as a powerful monotherapy for psoriasis, enabling many patients to achieve and sustain aggressive treatment targets. Its approval as a first-in-class topical therapy heralds a new era of personalised, effective, and well-tolerated options for managing plaque psoriasis.
Katie Wright, EMJ
Reference
Armstrong AW et al. Treat-to-target outcomes with tapinarof cream 1% in phase 3 trials for plaque psoriasis. Cutis. 2024;114(4):122-7.