Abstract
Background: The advent of JAK inhibitors (JAKi) has significantly modernised the treatment of atopic dermatitis (AD), offering a novel approach to treating this recalcitrant dermatological condition. Although topical treatment is shown to be effective, oral formulations are yet to be widely utilised in the treatment of AD.
Objectives: To review the efficacy, safety, and tolerability of JAKi in the treatment of AD.
Methods: A PRISMA systematic review of several databases was conducted: Cochrane Skin Specialised Register, Cochrane Central Register of Controlled Trials, Ovid Medline and Embase, LILACS, and Global Resource of EczemA Trials. Five clinical trial archives were also consulted. The following resources were manually searched: conference proceedings of the American Academy of Dermatology (AAD), FDA.gov, the European Medicines Agency (EMA), and Epistemonikos.
Results: Of the 34 articles meeting inclusion criteria, 6 were chosen for final qualitative review. A total of 827 patients were pooled from 5 randomised controlled trials and 1 cohort study. Improvements in objective and subjective scoring indices were observed in patients receiving topical or oral JAKi. Overall safety and tolerability were satisfactory in JAKi treatment.
Limitations: Due to the scarcity of randomised controlled trials and the small sample sets in the studies, a meta-analysis was not conducted.
Conclusions: Preliminary investigations show promising results for patients with AD treated with oral or topical JAKi. However, existing gaps should be addressed with more extensive and long-term trials before JAKi become a standard treatment for AD.
INTRODUCTION
Atopic dermatitis (AD) is one of the most common and debilitating chronic inflammatory skin diseases, often greatly affecting physical, economical, and psychological quality of life (QoL).1 It affects approximately 20% of children and 3–10% of adults,2 with mean lifetime prevalence increasing in recent decades.3,4 In 60% of cases, onset occurs in the first year of life; however, AD can present at any age.5 The course of AD ranges from chronic to relapsing-remitting, with 44% of cases spontaneously resolving in late childhood.6
AD is a clinical diagnosis with no definitive laboratory or histological findings. The hallmark of this condition is a disturbance of epidermal-barrier function due to recurrent skin inflammation, leading to dry skin, pruritis, and IgE-mediated allergen sensitisation.7 Skin lesions may then lead to increased risks of secondary bacterial and viral infections. Histologically, AD is characterised by skin infiltration with inflammatory cells, predominantly lymphocytes, eosinophils, and mast cells.8 AD is strongly associated with other atopic disorders, such as allergic rhinitis and asthma, with 50–80% of children exhibiting concurrent atopic manifestations.1 Other comorbidities of AD include cardiovascular disease, sleep disturbances, and cutaneous/systemic infections and malignancies, all of which highlight the correlation between inflammatory processes and atopic diatheses.
Treatment of AD is aimed at continuous epidermal-barrier repair through the use of emollients and avoidance of personal triggering factors.9,10 Topical corticosteroids, calcineurin inhibitors, and nonsteroidal topical phosphodiesterase-4 inhibitors are considered preliminary therapies for acute exacerbations. However, topical therapeutics for AD face many challenges, including imperfect efficacy, difficulty with application, adverse effects (AE) with long-term topical steroid regimens, and local site reactions.11 For severe cases of AD (modified Eczema Area and Severity Index [mEASI] score >10 with Investigator Global Assessment [IGA] >3 and >10% Body Surface Area [BSA] affected), phototherapy and systemic immunosuppressants (prednisone, cyclosporin, azathioprine, mycophenolate mofetil, or methotrexate) can be attempted. However, access to phototherapy is limited for many patients. Furthermore, side effect profiles for certain systemic immunosuppressants can decrease overall compliance.12 In 2017, dupilumab, an injectable monoclonal anti-IL-4Rα antibody, was successfully trialled in the USA for the treatment of moderate-to-severe AD.13 However, long-term data on its efficacy and safety are still pending.
The aetiology of AD is not yet fully clarified, but it is likely a multifactorial disease involving genetic and environmental components. Mutations in the filaggrin gene have been associated with AD and are thought to lead to epidermal barrier dysfunction.14 This dysregulation stimulates release of chemokines by keratinocytes15 causing subsequent immune cell infiltration, particularly Th2 cells and epidermal dendritic cells.16-18
Recently, JAK inhibitors (JAKi) have emerged as a novel therapeutic intervention for inflammatory diseases. JAK are intracellular secondary messengers that transmit extracellular cytokine signalling to the STAT pathway.19 There are four members of the JAK family: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). Cytokines that activate JAK have been implicated in lymphocyte activation and proliferation.20 Moreover, inhibition of the JAK–STAT pathway can suppress inflammation and inhibit immune-cell activation in T cell-mediated disorders.21 Currently, three JAKi have been approved in the European Union (EU): ruxolitinib and baricitinib (JAK1/2 inhibitors) are approved for myeloproliferative disorders (including polycythaemia vera) and rheumatoid arthritis (RA), respectively. Tofacitinib, a JAK1/3 inhibitor, is approved for RA, psoriasis, and ulcerative colitis. Novel selective JAK1 inhibitors, such as filgotinib, have also been efficacious in Phase IIa trials for RA.
Given the limited treatment arsenal for AD and the challenges posed by traditional topical and systemic agents, many patients are unable to achieve disease remission.22 Novel topical agents for AD have been absent for the past decade, making topical JAKi a promising option for recalcitrant disease. Although dupilumab is effective in the treatment of AD, its injectable formulation makes it prohibitive for certain patients. Given the optimistic safety profiles of oral JAKi and inconsistent compliance with topical agents, novel oral JAKi provide a meaningful alternative for patients afflicted with refractory AD.
METHODS
A search strategy was created on the basis of the Cochrane Handbook for Systematic Reviews of Interventions23 and the PRISMA statement.24 The authors’ review included randomised controlled trials (RCT), cohort studies, case reports and series, conference proceedings, and clinician-based experiences. Exclusion criteria included review articles, commentary pieces, patient-reported outcome studies, ongoing clinical trials, and preclinical investigations. No limitations were placed on language or publication status. The search strategy was peer reviewed by two independent librarians. The literature’s level of evidence was evaluated using The Oxford Centre for Evidence-Based Medicine (CEBM) Levels of Evidence Grading scale25 (Table 1).
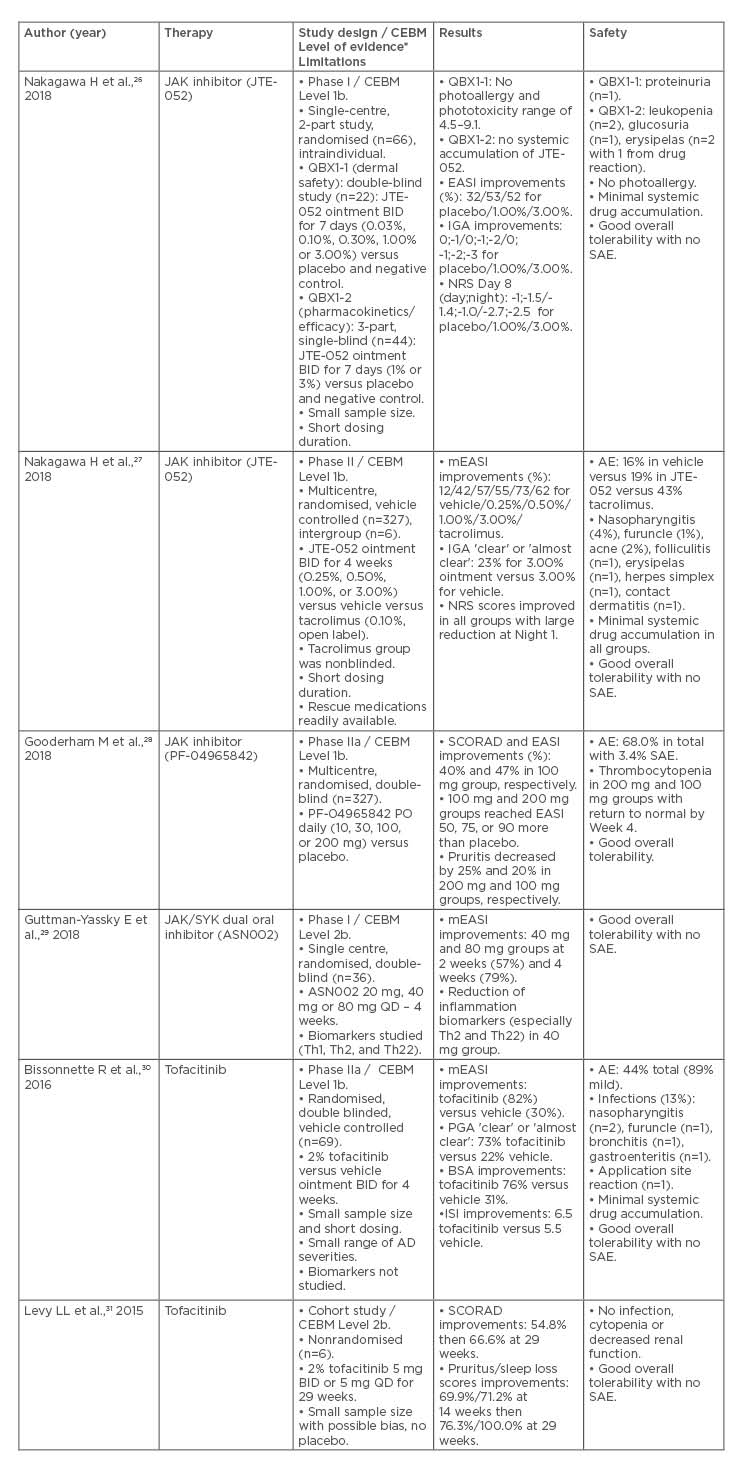
Table 1: Overview of current investigations in the treatment of atopic dermatitis with JAK inhibitors.
*The literature’s level of evidence was evaluated using The Oxford Centre for Evidence-Based Medicine (CEBM) Levels of Evidence Grading scale.25
AD: atopic dermatitis; AE: adverse event; BID: twice daily; BSA: body surface area; CEBM: Oxford Centre for Evidence-based Medicine; EASI: Eczema Area and Severity Index; IGA: Investigator’s Global Assessment; ISI: Itch Severity Item; JAK/SYK: JAK/spleen tyrosine kinase; mEASI: modified Eczema Area Severity Index; NRS: Numeric Rating Scale; PGA: Physician Global Assessment; PO: by mouth; QD: once daily; SAE: serious adverse event; SCORAD: Scoring Atopic Dermatitis.
Electronic Searches
The following electronic databases were systematically searched:
- Cochrane Skin Specialised Register (CRS)
- Cochrane Central Register of Controlled Trials (CENTRAL)
- MEDLINE via Ovid (from 1946 to 22nd June 2018) (Table 2)
- EMBASE via Ovid (from 1980 to 22nd June 2018) (Table 2)
- Latin American and Caribbean Health Science Literature (LILACS) Information database (from 1982 to 22nd June 2018)
- Global Resource of EczemA Trials (GREAT)

Table 2: Search strategies.
Complementary Resources
Clinical trial registers were manually searched (until 23rd June 2018), using the search terms “atopic dermatitis”, “eczema”, “neurodermatitis”, “Janus Kinase”, “JAK”, “Janus Kinase inhibitor”:
- International Standard Randomised Controlled Trials Number (ISRCTN) registry
- ClinicalTrials.gov
- Australian New Zealand Clinical Trials Registry (ANZCTR)
- World Health Organization (WHO) International Clinical Trials Registry Platform
- EU Clinical Trials Register
Conference Proceedings
- The American Academy of Dermatology (AAD)
Organisational Websites
- National Eczema Association (NEA)32
- U.S. Food and Drug Administration (FDA)33
- European Medicines Agency (EMA)34
- Epistemonikos35
RESULTS
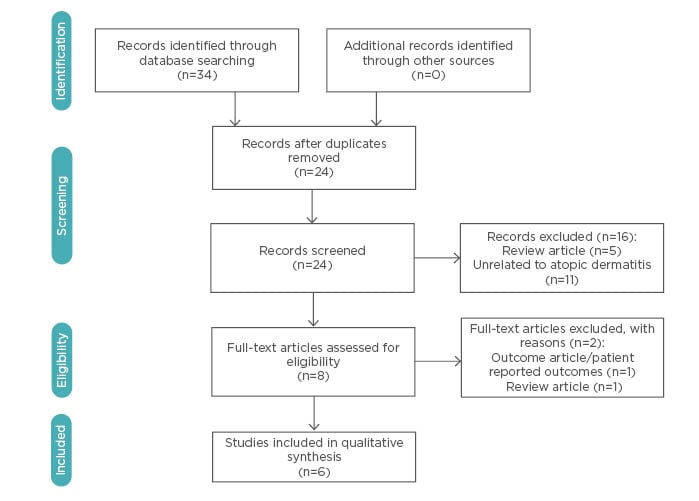
A comprehensive search yielded a total of 34 articles. Of these, six met the established inclusion criteria (Figure 1). A total of 827 patients were pooled from the 5 RCT and 1 cohort study identified. There were no case reports or case series singled out. A synthesis of the results was completed (Table 1).
Phase I Trial: Topical JTE-052 (JAK inhibitor)
Nakagawa et al.26 conducted a Phase I, single-centre RCT studying the efficacy, safety, and pharmacokinetics of a novel JAKi, JTE-052 (a JAK1/2/3 and TYK2 inhibitor), in the treatment of adult patients (18–65 years old) with AD. This RCT was divided into two subset studies: QBX1-1 and QBX1-2.
QBX1-1 investigated the cutaneous safety of JTE-052 ointment in 22 patients. A double-blind, randomised, intraindividual approach compared JTE-052 (0.03%, 0.10%, 0.30%, 1.00%, or 3.00% ointments) to placebo, white petrolatum ointments, and negative controls. Ointments were applied twice daily (BID) for 7 days (maximum of 5 g daily on any affected areas). Patch testing and photopatch testing were used to assess dermal safety at 60 minutes, 24 hours, then daily for 4 days. There were no positive photoallergy reactions in any of the 22 patients. The phototoxicity index ranged from 4.5 to 9.1 for the JTE-052 group compared to 4.5 for placebo, white petrolatum, and control groups. JTE-052 ointments up to 3.00% therefore showed a low potential for phototoxicity and no potential for photoallergy. The ointments were well tolerated with no serious AE or adverse drug reactions noted.
QBX1-2 studied the pharmacokinetics, efficacy, and safety of JTE-052 ointment in a single-blind, randomised analysis of 44 patients. JTE-052 ointment (1.00% or 3.00%) was applied BID for 7 days on patients with AD and healthy subjects. In Part 1, serial urine and plasma concentrations showed low systemic exposure and no systemic accumulation when 1.00% or 3.00% JTE-052 ointment was utilised. Exploratory efficacy was confirmed through improvements in several indices. Changes in EASI scores on Day 4 and Day 8 improved to 10.71%/32.71% in placebo, 30.99%/53.12% with 1.00% JTE-052, and 17.27%/52.26% with 3.00% JTE-052, respectively. Absolute changes in IGA on Day 8 were increased (improved) with 1.00% JTE-052 and 3.00% JTE-052 when compared to placebo. Absolute changes in pruritus Numeric Rating Scale (NRS) on Days 4 and 8 were -1.0/-1.0 (sleep) and -1.0/-1.5 (daytime) in placebo, -1.3/-1.4 (sleep), and -0.4/-1.0 (daytime), with 1.00% JTE-052 as well as -2.2/-2.7 (sleep) and -2.5/-2.5 (daytime) with 3.00% JTE-052. Overall tolerability and safety were good, with one case each of glucosuria (placebo group), leukopenia (3.00% JTE-052), and erysipelas (1.00% JTE-052).
Phase II Trial: Topical JTE-052 (JAK inhibitor)
A Phase II multicentre, intergroup, vehicle-controlled RCT was conducted by Nakagawa et al.27 as a follow-up to their Phase I trial. This study was not double-blinded in both groups; however, site personnel handled all samples with both investigators and patients unaware of the appearance of the ointments. The efficacy, safety, and pharmacokinetics of JTE-052, a novel JAKi, were investigated across 38 centres in 327 patients with moderate-to-severe AD.
JTE-052 ointment (0.25%, 0.50%, 1.00%, or 3.00%), the vehicle ointment, or tacrolimus (0.10%) ointment was applied BID for 4 weeks (6 groups total). There were no baseline differences in the severity of AD amongst patients. At end of treatment in Week 4 (or at study discontinuation), all groups showed a decrease in mEASI (p<0.001 for JTE-052 at all concentrations). Reduction in mEASI was dose-dependent, with mean changes of -12.2%, -41.7%, -57.1%, -54.9%, and -72.9% for vehicle 0.25% and 0.50%, 1.00%, and 3.00% JTE-052, respectively. The mean change in mEASI for the tacrolimus group was -62.0%. Improvements in IGA, pruritus NRS, and percentage BSA were also noted in all JTE-052 groups over time. The proportion of patients achieving an IGA score of ‘clear’ or ‘almost clear’ was higher in 3.00% JTE-052 group (23%) compared to the vehicle group (3%) (p=0.039). Of note was the rapid antipruritic effect of 0.5% JTE-052 (with improvements proportional to dosage concentration) from the first night of application versus vehicle (p=0.001). No statistical comparisons between tacrolimus and JTE-052 were performed in regard to antipruritic effects. At Weeks 2 and 4, plasma concentrations of JTE-052 were highest in the 1.00% and 3.00% JTE-052 groups. Minor AE (mostly skin infections) were reported in 16.0% of patients in the vehicle group compared to 19.2% in the JTE-052 groups. The tacrolimus group was associated with the highest proportion of application site reactions. Overall tolerability of application was good in all groups.
Phase IIb Trial: Oral PF-04965842 (JAK inhibitor)
A Phase IIb trial was conducted by Gooderham et al.28 to examine the secondary efficacy and safety of PF-04965842, a novel oral JAK1 inhibitor. This double-blind, multicentre RCT followed 323 patients over 12 weeks. Patients were administered 10, 30, 100, or 200 mg of PF-04965842 versus daily placebo by mouth (PO). Scoring of AD index (SCORAD) and EASI scores improved by 40.7% (p=0.0017) and 47.4% (p=0.0091) in the 100 mg group compared to placebo, respectively. Patients in the 100 mg and 200 mg groups achieved EASI 50, 75, or 90 more often than with placebo (EASI50: 78.5% and 55.3% versus 27.4% [p=0.0042]; EASI75: 63.7% and 41.6% versus 15.6% [p=0.0043]; EASI90: 51.6% and 26.8% versus 10.3% [p=0.0354]). Placebo-adjusted percentage change from baseline for pruritus severity was 25.4% (p=0.0034) in the 200 mg and 20.7% (p=0.0172) in the 100 mg group. PF-04965842 was generally well tolerated, with 68.9% AE and 3.4% serious AE (thrombocytopenia).
Phase I Trial: Oral ASN002 (JAK/spleen tyrosine kinase inhibitor)
A Phase I trial was conducted by Guttman-Yassky et al.29 to investigate the tissue response, safety, and clinical efficacy of ASN002, a novel dual oral inhibitor of JAK/spleen tyrosine kinase (JAK/SYK) signalling. JAK/SYK (including TYK2) signalling controls AD related Th2 and Th22 cytokine production (suppressing IL-6 and IgE stimulation). This double-blind RCT followed 36 patients with moderate-to-severe AD. ASN002 20 mg, 40 mg, 80 mg doses, or placebo were administered daily (QD) for 4 weeks. Skin biopsies were evaluated at baseline, 2 weeks, and 4 weeks for biomarkers. Overall, amongst the 40 mg and 80 mg ASN002 groups, optimal mEASI score improvement occurred at 2 weeks (57% change) and 4 weeks (79% change). Reductions in inflammation, T-cell activation, hyperplasia, Th2/Th22, and Th1 were noteworthy in the 40 mg ASN002 group (p<0.004). A correlation was also noted between improvements in EASI and Th2/Th22 biomarkers. Overall, there was adequate tolerability and safety for product administration in all groups.
Phase IIa Trial: Topical Tofacitinib (JAK inhibitor)
Bissonnette et al.30 completed a Phase IIa, double-blind, parallel-group, vehicle-controlled, multicentre RCT in 69 adults with moderate-to-severe AD. The efficacy, safety, and pharmacokinetics of 2% tofacitinib ointment (JAKi) was evaluated via a BID regimen over 4 weeks. After 4 weeks, improvement in mEASI was greater in the tofacitinib group (81.7%) compared to the vehicle group (29.9%) (p<0.001). Similarly, the proportion of patients with a Physician Global Assessment (PGA) of ‘clear’ or ‘almost clear’ at Week 4 was 73% in the tofacitinib group compared to 22% for the vehicle group (p<0.05). At 4 weeks, a 76% improvement from baseline BSA was seen in the tofacitinib group compared to 31% in the vehicle group (p<0.001). Improvements in the baseline Itch Severity Item (ISI) score were greater in the tofacitinib group (6.5) versus the vehicle group (5.5) (p<0.001). Overall, 44% of the patients experienced AE, of which 89% were mild. The tofacitinib group included 12 AE (in 11 patients) compared to 26 AE (in 19 patients) for the vehicle group. Two patients in the vehicle group discontinued treatment because of AE. The most frequent AE were infections and infestations (13%). Postadministration plasma tofacitinib concentrations in Weeks 2 and 4 were only slightly higher than pre-dose concentrations, indicative of a flat concentration curve. Concentrations increased with higher treated percentage BSA at Week 2 but not Week 4.
Cohort Trial: Oral Tofacitinib (JAK inhibitor)
Levy et al.31 evaluated the efficacy of oral tofacitinib citrate (a JAK1/3 inhibitor) in 6 consecutive patients (18–55 years old) with refractory AD. Moderate-to-severe AD was established with a baseline SCORAD of >20. Over 29 weeks, 5 patients received 5 mg (PO) BID and 1 patient received 5 mg (PO) QD (since QD dose was sufficient to elicit remission). Assessments were conducted at 4 to 14 weeks then 8 to 29 weeks. In all six patients, tofacitinib treatment resulted in reduced dermatitis and oedema BSA score. Composite SCORAD index decreased by 54.8% from Weeks 4 to 14 and decreased by 66.6% compared to baseline at Week 29 (p<0.05 for all comparisons). At Week 14, the pruritus and sleep loss scores decreased by 69.9% and 71.2%, respectively (p<0.05). These scores diminished by 76.3% and 100.0% from baseline at Week 29. Oral tofacitinib was well tolerated overall, with few AE reported.
DISCUSSION
Recent developments in the study of topical and oral JAKi have greatly advanced the understanding of AD and its response to novel treatment alternatives. The authors’ review bridges the gap between previous knowledge and current concepts addressing the use of JAKi in AD. The majority of the studies captured in this review describe Phase I26,28 or Phase II27,29 clinical trials. Completed Phase III data is currently unavailable, although multiple adult and paediatric clinical trials studying novel JAKi (including oral baricitinib, topical tofacitinib, and oral upadacitinib) are under way in the USA and Europe.36-43 Both topical and oral JAKi resulted in reductions in AD disease severity compared to placebo/vehicle. Marked and rapid reductions were observed for most pruritus scores,26,30 sometimes within 1 day of initiating treatment. Overall, safety, tolerability, and systemic accumulation of JAKi (via measurement of urine and plasma concentrations) were well within acceptable ranges. Aggregate findings therefore suggest that both oral and topical JAKi are safe and efficacious in the treatment of AD.
The success of JAKi in controlling AD also confirms the importance of the JAK–STAT pathway in the pathogenesis of the disorder. Cytokines such as IL-4, which increase in AD, make use of JAK for signalling.44,45 IL-4 promotes differentiation of Th2 cells, and subsequent production of other inflammatory cytokines (IL-4, IL-5, IL-10, and IL-13). Given that AD is overwhelmingly a Th2 focussed disorder, JAKi are a promising treatment option for AD.
Of the JAKi that were examined in this present review, tofacitinib was most extensively studied in major inflammatory conditions, including immune-mediated dermatologic conditions.42-44 Tofacitinib preferentially blocks signalling through JAK1 or JAK3 which are paired with JAK2.49,50 Several cytokines, including IL-4, signal through this pathway21 whereas IL-13 signals through JAK1/TYK2. The authors identified a Phase IIa study using topical tofacitinib30 and a cohort study using oral tofacitinib.27 Though topical tofacitinib has conflicting efficacy for plaque psoriasis,51,52 the Phase II trial included in this present review showed it to be superior to placebo29 for the treatment of AD. Oral tofacitinib can also safely lead to clearance of moderate-to-severe AD.30 However, in this study, success was demonstrated in a small, noncontrol cohort study (n=6), which may limit extrapolation to the general population.
Three novel JAKi were also efficacious in the treatment of AD. In vitro, JTE-052 inhibited JAK1, JAK2, and JAK3.53 In animal dermatitis models, activation of inflammatory cells, was inhibited consequently supressing skin inflammation.54 JTE-052 also successfully inhibited keratinocyte production of filaggrin,55 a contributor to the pathogenesis of AD. Accordingly, Phase I and Phase II studies26,27 showed that topical JTE-052 was superior to placebo in reducing disease severity and pruritis. Finally, PF-04965842 (oral JAK1/2 inhibitor) and ASN002 (oral dual JAK/SYK inhibitor) showed promising results in Phase I and Phase II RCT, respectively.28,29 ASN002 also manifests strong antitumor properties, suppressing haematological malignancies in preclinical studies.56
Pruritis is a major feature of AD and leads to a significant reduction in QoL,57 often analogous to the discomfort experienced in chronic pain syndromes. IL-31 plays a lead role in the pruritis pathway for patients with AD.21 Previous studies demonstrated that tofacitinib and JTE-052 may suppress IL-31.58-60 This was supported by the rapid and significant reduction in pruritis observed during the Nakagawa et al.26,27 Phase I/II trials.
JAKi are also involved in pathways that are important for immunity. This has led to concerns regarding the effects of JAKi in immune and haematopoietic development.61 The JAKi that the authors reviewed exhibited a low incidence of AE, most of which were mild in severity. There was no clear dose-related association to AE; additionally, incidence and severity of AE were not attributed to particular JAKi or formulations (oral versus topical). Of note, one study showed higher rates of AE in vehicle groups when compared to JAKi groups.29 Most AE were infectious in nature (nasopharyngitis, bronchitis, furuncle, gastroenteritis, and viral upper respiratory tract infections). An event of erysipelas (outside of the application area) with 1% JTE-052 ointment26 was deemed a drug-related AE, potentially attributable to JAK inhibition. Given short study durations and limited samples sizes, inferences regarding the long-term safety of JAKi cannot be presently established. One of the limitations of this review was therefore the inability to conduct a meta-analysis because of the shortage of RCT and the small sample sets in the studies. The exposure histories were not thoroughly investigated between study groups in each trial; however, the efficacy and safety of tofacitinib is evident in other inflammatory diseases such as RA62,63 and ulcerative colitis.64 Murine models are at risk of latent tuberculosis reactivation61 with cases reported in trials of tofacitinib in RA patients.66 Therefore, the efficacy of JAKi should be weighed against black box warnings such as serious infections and malignancies. In RA trials, tofacitinib treatment was associated with dose-dependent decreases in mean neutrophil counts and haemoglobin, with normalisation of blood counts during the treatment period without intervention.67 In psoriasis, alterations in blood lipid profiles were also seen in some patients using tofacitinib.68,69 Although the short-term safety profiles of tofacitinib and other JAKi reported in this review were acceptable, data should be interpreted with caution, especially if extrapolating to long-term treatment regimens.
CONCLUSION
JAKi remain a promising new therapeutic modality for patients with AD. Traditional topical agents, such as corticosteroids and calcineurin inhibitors, have historically poor adherence and a higher incidence of application site reactions. Given their established efficacy, low rate of AE, and rapid relief of pruritis, continued investigations into topical JAKi for the treatment of AD should be thoroughly undertaken. Although only two studies in this review examined the efficacy and safety of oral JAKi, the convenience and potential improved adherence of oral agents make them a realistic alternative in the treatment of AD. However, continued explorations into the efficacy and long-term safety of JAKi should be addressed by means of more extensive Phase III/IV clinical trials.







