Abstract
Platelet-rich plasma (PRP) is autologous plasma with a platelet concentration that is on average five times greater than baseline, and has been extensively investigated for its potential applications across various medical fields, including orthopaedics, dermatology, wound healing, maxillofacial surgery, and others. This review article aims to provide an overview of PRP’s applications and evidence over the past 5 years in randomised controlled trials. Many studies demonstrate PRP’s effectiveness in reducing pain and improving functional outcomes, while others report no significant differences compared to alternative treatments or placebo. Across various studies, several key limitations exist, such as small sample sizes, short follow-up durations, and lack of standardisation in PRP preparation methods, highlighting the need for research to further establish PRP’s effectiveness in these clinical applications. The article also discusses the different classification systems for PRP, and underscores the importance of understanding the components that influence clinical outcomes, as well as noting the growth of PRP in the marketplace. Overall, while studies exist demonstrating the clinical utility of PRP, standardised reporting is required to determine its full potential, as well as optimal preparation and administration strategies.
Key Points
1. This manuscript provides an overview of randomised controlled trials on the therapeutic po-tential of platelet-rich plasma (PRP) in various medical fields, such as orthopaedics, dermatology, and maxillofacial surgery. Here, the authors reveal PRP’s varying effectiveness in reducing pain, improving functional outcomes, and contributing to tissue regeneration, underlining its growing prominence in the medical marketplace.2. This review underscores the prevailing challenges in PRP research, including small sample sizes, short follow-up durations, and a lack of standardisation in preparation methods. The manuscript emphasises the need for accurate documentation of PRP preparation aspects, such as growth factor levels, to facilitate cross-study comparisons, and address the heterogeneity in PRP prepa-ration and administration strategies, which currently hinder the comparability of PRP’s efficacy among different studies.
3. There is a need for further research to establish and normalise standardised reporting, optimal preparation, and administration strategies for PRP. Given the substantial growth of the PRP global market, and its increasing usage, the authors call for blinded randomised controlled trials, and robust characterisation of PRP’s constituents to fully assess its efficacy, and to inform clinicians on its optimal utilisation for diverse medical applications.
INTRODUCTION
Platelets, small anucleate cells derived from megakaryocyte fragments that play a central role in forming blood clots during vascular and tissue injury to maintain homeostasis.1 These cells harbour three types of granules: dense, α, and lysosomal granules. Specifically, the α granules are repositories of a host of bioactive factors, such as growth factors, cytokines, chemokines, cell adhesion molecules, and proteins.1,2
Platelet-rich plasma (PRP), a concentrate of these bioactive constituents, is essentially a cocktail of growth factors, cytokines, and other molecules. PRP’s therapeutic potential has been progressively explored across multiple medical specialties, including musculoskeletal, dermatology, chronic wound healing, and maxillofacial surgery, among others (Table 1).
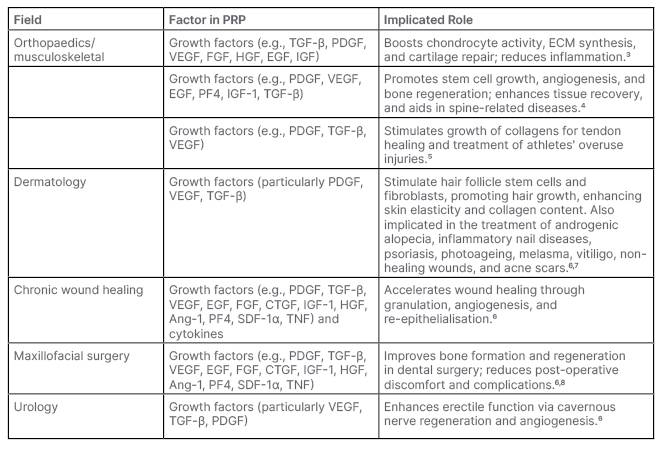
Table 1: Breakdown of frequently cited growth factors and their functions in each major field of platelet-rich plasma use.
Ang-1: angiopoietin-1; CTGF: connective tissue growth factor; ECM: extracellular matrix; EGF: epidermal growth factor; FGF: fibroblast growth factor; HGF: hepatocyte growth factor; IGF: insulin-like growth factor; PDGF: platelet-derived growth factor; PF4: platelet factor 4; PRP: platelet-rich plasma; SDF-1α: stromal cell-derived factor 1; VEGF: vascular endothelial growth factor.
The clinical utility of PRP dates to the 1970s, and its use has since pervaded several fields, such as orthopaedics/musculoskeletal, dermatology, chronic wound healing, maxillofacial surgery, ophthalmology, urology, and others (Figure 1). This prevalence of PRP applications has also driven a robust growth in its global market, valued at 627.9 million USD in 2022, with a projected annual growth rate of 15.1% compounded yearly from 2023–2030.9
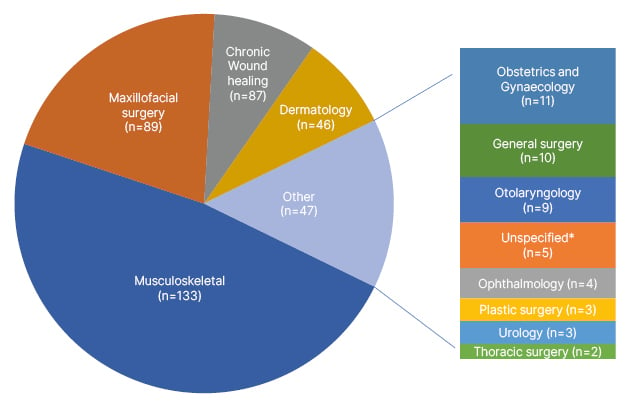
Figure 1: Breakdown of randomised controlled trials on PubMed over the past 5 years using platelet-rich plasma (n=402).
*Unspecified areas in “Unspecified” category include: pain management and rehabilitation (n=2), rheumatology (n=1), diagnostic medicine (n=1), and neurology (n=1).
Data collected from a PubMed Medical Subject Heading “Platelet-Rich Plasma” search over the last 5 years, including only randomised controlled trials, and updated on 11th January 2023.
PRP is obtained through a centrifugation process that separates whole blood into distinct layers, comprising of erythrocytes at the bottom, the buffy coat in the middle, and plasma at the top (Figure 2).10 The buffy coat layer, rich in platelets and leukocytes, is not uniform, and can be stratified into sub-layers containing different cell types and platelet concentrations. Closer to the erythrocyte layer, there is typically a high concentration of neutrophils and other granulocytes, alongside a moderate number of platelets. As you ascend towards the upper portion of the buffy coat layer, closer to the plasma, platelet concentration tends to increase, while an increase in mononuclear cells, such as lymphocytes and monocytes, is observed. A standardised nomenclature system, such as Dohan Ehrenfest’s shorthand naming convention, categorises different types of PRP, primarily based on the depth of buffy coat collection (Figure 2).
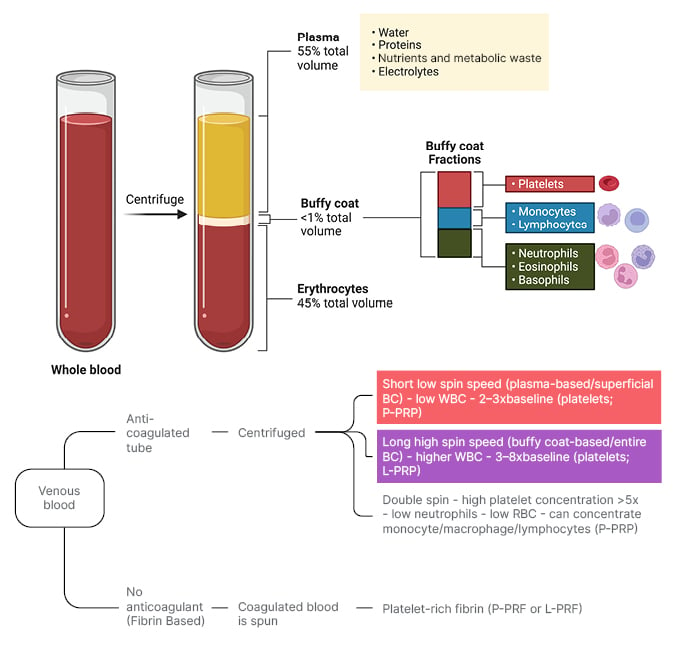
Figure 2: Platelet-rich plasma collection method.
Top half: Whole blood, pre- and post-centrifugation, with separated layers and buffy coat layers labelled.
Bottom half: Collection methods of PRP as they pertain to the Dohan Ehrenfest shorthand naming convention of PRP. Superficial buffy coat collection (red box) as it pertains to P-PRP and P-PRF, and entire buffy-coat collection (purple box) as it pertains to L-PRP and L-PRF production.
BC: buffy coat; L-PRF: leukocyte- and platelet-rich fibrin; L-PRP: leukocyte- and platelet-rich plasma; P-PRF: pure platelet-rich fibrin; PRP: platelet-rich plasma; P-PRP: pure platelet-rich plasma; RBC: red blood cell; WBC: white blood cell.
Figure created with BioRender.com.
The therapeutic efficacy of PRP, especially in tissue regeneration, is often tied to the release of its growth factors and other bioactive molecules. To achieve an immediate release of these factors, PRP can be activated. This is typically achieved through the addition of exogenous coagulation factors, such as thrombin or calcium chloride.11,12 This release, when induced externally by such coagulants, can span from minutes to days. Alternatively, a ‘physiologic activation’ method can be employed. In this method, PRP, when injected, undergoes natural activation, leading to a more prolonged, steady release of platelet factors over 1–2 weeks.11,12 It is worth noting that the choice of activation process can influence the therapeutic potential of PRP. External activation provides an immediate high concentration of growth factors, which might be beneficial in scenarios where a rapid tissue response is desired. In contrast, the slower physiologic activation might be more suitable for sustained release applications. Notwithstanding the widespread use of PRP, its preparation protocols and standardisation to attain a consistent optimal platelet yield remain a matter of discussion.
While a comprehensive understanding of the crucial elements that drive the benefits of PRP in various fields often remains elusive in literature, attempts are frequently made to conjecture the contributing factors to the benefits observed with PRP use. Within orthopaedics, for instance, it is postulated that growth factors stimulate chondrocyte activity, extracellular matrix synthesis, and cartilage repair, and alleviate inflammation.3-7 These growth factors typically form the basis of PRP’s reported benefits across numerous fields (Table 1). However, a consistent depiction of this foundational role in literature is often complicated, due to the lack of formal growth factor analyses in studies demonstrating benefits with PRP use.
Given the intricate nature of PRP’s constituents, preparation methods, and wide-ranging applications in various medical fields, this review aims to collate and analyse current findings on PRP’s therapeutic utility, while identifying gaps in the literature regarding standardisation, growth factor analyses, and efficacy reports. By synthesising domain-specific data and outcomes, the authors hope to shed light on the mechanisms underlying PRP’s therapeutic benefits, and underscore areas requiring rigorous scientific investigation.
METHODS
The review of the literature was conducted following a structured approach to ensure the inclusion of all relevant studies and data regarding the clinical use of PRP across diverse medical fields. Emphasis was laid on the most frequent use cases and documented effectiveness. The search, initiated on 11th January 2023, focused on randomised controlled trials (RCT) from the past 5 years. The electronic database PubMed was utilised, employing the Medical Subject Heading term “Platelet-Rich Plasma” to yield 402 pertinent articles.
Post-retrieval, abstracts of the identified articles were examined for relevance, followed by a detailed review of the full-text versions. Studies were then stratified into distinct thematic groups based on their outcomes, enabling a comprehensive summary of each field. Further, each study was critically assessed for limitations, contributing to the formation of summary groups.
The most extensive and representative studies from each summary group were selected, ensuring a balanced depiction of both the outcomes and limitations within each field of PRP use. This approach aimed to create an inclusive, yet concise, review that encapsulates the breadth of PRP’s clinical application and efficacy.
MUSCULOSKELETAL (n=133)
Knee Osteoarthritis
Studies on PRP’s efficacy in treating knee osteoarthritis (KOA) compared versus placebo with normal saline, or against widely accepted treatments, such as injections of hyaluronic acid (HA) or corticosteroids, have reported mixed results.13,14
An RCT aimed to compare the clinical outcomes of intra-articular injection of PRP and high molecular weight HA in treating KOA. In this study 200 patients with symptomatic KOA (Kellgren–Lawrence Grade 2 or 3) received three blinded intra-articular injections, at 2-week intervals, of either PRP or HA. Clinical evaluations employed metrics like the International Knee Documentation Committee (IKDC) subjective score, Visual Analogue Scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total score, and the re-injection rate over a 36-month follow-up period. By the final evaluation, 189 patients remained in the study.
PRP and HA injections both effectively improved knee symptoms and functional status, with results stable up to 18 months post-injection without any re-injections. Beyond the 18-month period, the PRP group showed a decrease in mean vasscores, reflecting a sustained reduction in pain. Simultaneously, the IKDC subjective scores improved, indicating enhanced knee function over time. WOMAC total scores also decreased over the study period, suggesting overall amelioration in pain, stiffness, and physical function. In contrast, the HA group demonstrated initial improvement followed by a gradual decline in IKDC subjective scores, suggesting a deterioration in knee function. The VAS scores for this group also fluctuated, ultimately increasing, and indicating worsening pain symptoms.
The re-injection rate was notably lower in the PRP group during the 24–36-month follow-up period, highlighting PRP treatment’s longer-lasting effect. Despite the higher incidence of short-term post-injection pain in the PRP group, no major complications were associated with either injection type.
In contrast, another study found the benefits of PRP were more aligned with HA.15,16 Specifically, in a double-blind study involving 192 patients observed over an average of 64.3 months (standard deviation [SD]: 7.8 months), both PRP and HA, administered via three weekly injections, led to comparable improvements in IKDC subjective scores, with significant advancements from baseline for both treatments. The only notable difference was a reduced reintervention rate in the PRP group at 24 months (22.6% versus 37.1%; p=0.036).15
Temporomandibular Joint Osteoarthritis
Within the realm of temporomandibular joint osteoarthritis (TMJ-OA) treatments, an RCT delved into the benefits of intra-articular platelet-rich fibrin (PRF) injections following arthrocentesis. Notably, PRF, produced sans anticoagulants, allows a fibrin clot formation during centrifugation, serving as a cellular migration scaffold, and promoting tissue healing. In a study population of 36 patients, equally divided into a PRF group and a control group, the PRF cohort exhibited marked improvements in jaw movement metrics through the 6th month post-treatment, and these positive outcomes persisted until the 12th month. In contrast, the control group, which only underwent arthrocentesis, demonstrated an initial post-operative uptick in jaw movement measurements, but this trend reversed between the 6th and 12th month. Nevertheless, pain levels notably declined in the control group (p<0.001). The overarching inference drawn was the superiority of PRF injections after arthrocentesis over the latter in isolation, in terms of pain alleviation and bolstered functional jaw motions. 17
Shifting focus to a clinical and radiological study conducted in 2019, the impact of PRP, HA, and corticosteroids on TMJ-OA was explored. The research encompassed 31 patients experiencing lateral pain, and 43 patients with posterior pain, grouped into three cohorts: PRP (Group 1), HA (Group 2), and corticosteroids (Group 3). The findings illuminated that PRP intra-articular injections were notably superior in alleviating TMJ palpation pain when juxtaposed with HA and corticosteroids, as gauged through the VAS scores at varied post-treatment intervals.18
In a more recent RCT from 2022, researchers scrutinised the potency of PRP, HA, and a hybrid of both, succeeding arthrocentesis in 30 patients with TMJ-OA. The outcomes showcased a prominent pain diminution across all cohorts 6 months post-treatment, with the blend of PRP and HA emerging as the most efficacious (p<0.001). Furthermore, parameters such as the maximum mouth opening and both lateral and protrusive mandibular movements witnessed a commendable surge in all tested groups, with the zenith observed in the combined treatment cohort.19
Rotator Cuff Injuries
Research into rotator cuff injuries has elucidated PRP’s potential role. A double-blind RCT assessed PRP versus sodium hyaluronate, either standalone or combined. Using metrics such as the Constant score and the American Shoulder and Elbow Surgeons (ASES) Shoulder Score, results indicated a combined sodium hyaluronate and PRP treatment to be especially beneficial for specific rotator cuff injuries.20
Another RCT compared corticosteroid injections to PRP. While corticosteroids provided initial relief, PRP shone in long-term benefits, demonstrating its potential for sustained recovery in rotator cuff injuries.21
A similar double-blind RCT juxtaposed PRP and corticosteroid injections. The results illuminated that 3 months post-injection, the PRP group manifested noticeable improvements in VAS, ASES, and Western Ontario Rotator Cuff Index scores, though these differences were not significant at the 6-week or 12-month intervals.22
One study evaluated the effectiveness of collagen with PRP, PRP alone, and collagen alone in treating partial-thickness rotator cuff injuries. Utilising a numeric rating scale, QuickDash, and EQ-5D-5L questionnaires for assessment, they found no significant differences between the groups. However, a trend towards improvement was observed in the combined collagen and PRP group and PRP alone group between the 12th and 24th week follow-ups.23
In summary, the realm of musculoskeletal applications has seen varied PRP outcomes. While certain studies emphasise its potential in pain mitigation and functional improvement, such as in TMJ-OA cases,17-19 others present ambiguous results.13-18,24,25 The discrepancies in outcomes can be attributed to variations in sample sizes, follow-up durations, and PRP preparation methods, highlighting the necessity for more meticulous research in the field.
MAXILLOFACIAL SURGERY (n=89)
The effectiveness of PRP and PRF in maxillofacial surgery has been the focus of various studies, yielding mixed results. Some studies demonstrated the benefits of PRP and PRF in surgical outcomes, such as reduced scar width; improved patient satisfaction and quality of life;26 enhanced recovery of neurosensory disturbances following sagittal split osteotomy (SSO);27 and increased tooth movement rate with injectable PRF, potentially shortening orthodontic treatment.28 PRP is also shown to improve pain alleviation and mouth opening in patients with temporomandibular joint derangement,29 while PRF has shown potential in reducing palatal wound dehiscence in relocation pharyngoplasty for patients with obstructive sleep apnoea.30
In a study of 21 patients with bilateral SSO (15 females, 6 males; average age: 25.48±5.16 years), PRF was evaluated for its potential to hasten recovery from paraesthesia post-operatively. The study analysed the Two-Point Discrimination (TPD) value, brushstroke direction, and self-reported paraesthesia via the VAS. PRF treatment resulted in a significant reduction in TPD values at both 6- and 12-months post-operation compared with the control group (p=0.001). Similarly, a significant increase in correct brushstroke direction reporting was observed in the treatment group at both timepoints (p=0.001). Moreover, VAS paraesthesia scores were significantly lower in the PRF group at both 6 and 12 months (p=0.001). The results suggest that PRF may expedite paraesthesia recovery after SSO.
At 6 months post-surgery, Group 1 (treatment) showed a mean TPD value of 6.33±0.66 mm, while Group 2 (control) were at 7.29±0.72 mm, with the difference being statistically significant (p=0.001). Similarly, at 12 months, the treatment group’s mean TPD value was lower than the control’s (4.71±0.78 mm versus 6.19±0.75 mm; p=0.001). In the brush directional stroke test, significantly more patients in the treatment group were able to report the correct direction at both 6- and 12-months post-surgery (81.0% versus 28.6% and 100.0% versus 71.4%, respectively; p=0.001). Finally, the self-reported paraesthesia VAS score was also significantly lower in the treatment group at both timepoints (5.62±0.59 versus 7.00±0.70 at 6 months, and 3.52±0.68 versus 4.95±0.59 at 12 months, respectively; p=0.001). These findings indicate that PRF application may enhance the recovery of paraesthesia following SSO.27
Furthermore, PRF has been found to be effective in promoting bone regeneration around immediate dental implants, with primary stability measured between 30 and 60 on the Implant Stability Quotient (IQS) scale,31 and preventing post-operative relapse after Le Fort I osteotomy.32 A combination of bone marrow aspirate concentrate and PRF has been suggested to result in more mature bone formation,33 and PRF alone could enhance the stability of dental implants in the posterior maxilla during the healing period.34
In contrast, other studies found no significant benefits of PRP and PRF in certain applications. For example, PRP showed no healing effects on alveolar defects after rapid maxillary expansion,35 and liquid PRF did not significantly affect implant surface osseointegration.36 Despite some positive findings, many of these studies had limitations, such as small sample sizes and lack of blinding. Therefore, further research is needed to confirm the conclusions, and better understand the role of PRP and PRF in maxillofacial surgery.
WOUND HEALING (n=87)
Chronic wounds do not progress through the normal phases of healing, and can be classified as vascular, diabetic, or pressure ulcers.37 Their prevalence in the USA has created an economic burden on the healthcare system.38 The treatment of chronic wounds is summarised by TIME: Tissue debridement, Infection control, Moisture balance, and Edges of the wound.37 Further management involves treatment and control of the underlying disease.37
PRP has emerged as a promising adjuvant for the treatment of chronic wounds. The α granules of platelets contain growth factors, including transforming growth factor (TGF-β1, TGF-β2), granulocyte colony-stimulating factor, TNF-α, platelet-derived growth factors, platelet-derived angiogenesis factor, keratinocyte growth factor, hepatocyte growth factor, and insulin-like growth factor, which are released upon platelet activation.39 Growth factors play a vital role in the wound healing process.
The effectiveness of PRP therapy on the management of chronic wounds has been evaluated on various types of chronic wounds, as well as on different end outcomes in regard to wound healing.40-42 Commonly used end outcomes include the size of the wound, time to complete closure of the wound, hospitalisations, and infections. Most of the studies found PRP provided a benefit in at least one end outcome. A double blind RCT aimed to assess the efficacy of platelet gel compared to hydrogel in the treatment of non-healing chronic lower leg ulcers of different aetiologies.43 Thirty patients were treated with platelet gel, and 30 with hydrogel once a week for 3 consecutive weeks, with a last examination 6 months after treatment. They found that after 6 months of treatment, the mean wound area of the experimental group treated with platelet gel decreased to 35.01% (SD: 53.69). The control group (treated with hydrogel) had the wound area decrease to 89.95% (SD: 71.82). Overall, the treatment with platelet gel was statistically significantly more efficacious than hydrogel (p<0.05). Another study analysed diabetic foot ulcers treated with PRP, and reported a higher healing rate than patients treated with conventional dressing.44 One study by Gupta et al.41 found diabetic foot ulcers treated with PRP did not have a better or faster healing than conventional treatment. In the study, patients randomised to receive normal saline dressing had an 81.72±17.2% mean±SD percentage reduction in healing area at 6 weeks compared to 85.98±13.42% in the study group (p=0.29). A limitation of some of these studies include testing on chronic wounds of various aetiologies, which may have influenced the results. Overall, as platelets contain growth factors that play a significant role in wound healing, the use of PRP demonstrates success in improving end outcomes of the wound healing process; however, further studies are necessary to further establish this effect.
DERMATOLOGY (n=46)
PRP has been effective in several dermatologic diseases, including alopecia, melasma, scars, and vitiligo.45-47 The biggest use has been on patients suffering from androgenetic alopecia (AGA).48,49 Specifically, over the past 5 years, 21 RCTs attempted to assess the efficacy and safety of PRP for the treatment of AGA. Many studies found PRP to be beneficial; however, there are key limitations. A split-scalp study enrolled 35 patients with AGA to assess the effects of PRP on hair growth and thickness. Two 7.6 cmx7.6 cm squares were tattooed on the scalp of participants, who were randomly assigned to PRP or saline injections of three monthly treatment sessions, and an evaluation 3 months after the final treatment session. The PRP treated areas exhibited a mean density increase of approximately 20 hairs/cm2 (p<0.05).50 The placebo side showed a mean density increase of approximately 15 hairs (<0.05). The authors attribute the increase in hair density in the placebo side to the possibility of growth factors diffusing from the PRP-treated areas, therefore leading to an improvement in both the control and PRP-treated sides of the scalp. Additionally, the process of injecting needles into the scalp can lead to the immobilisation of growth factors, which can contribute to improved hair growth. These factors make difficult an effective assessment of the efficacy of PRP in split- scalp studies.
PRP has demonstrated efficacy in vitiligo treatment. There have been five RCTs published in the last 5 years on the use of PRP for the treatment of vitiligo. Four of these studies assessed the efficacy of PRP in addition to laser therapy. All but one found that PRP can be an effective adjunct for treating vitiligo. One study explored the efficacy of PRP versus combined fractional CO2 laser with PRP in the treatment of non-segmental vitiligo. The study enrolled 36 participants who were treated with PRP, fractional CO2, combined PRP, and fractional CO2; and a fourth control group. The study demonstrated that PRP alone provided the best results, followed by PRP with CO2, and then fractional CO2 alone.51 Specifically, the mean surface area reduction of the PRP treated area was 57.01+/-29.67, for the CO2 and PRP, a mean reduction of 54.22+/-37.08 was observed and for Co2 alone, a reduction of 38.08+/-40.32. A study aimed to assess whether the addition of PRP to monochromatic excimer light (MEL) therapy would provide additional benefit in the treatment of localised stable vitiligo. The study enrolled 36 patients with at least two more or less symmetrical patches of localised and stable vitiligo. For each participant, each vitiligo patch was randomly assigned to receive either MEL therapy combined with PRP injections (Group A), or MEL therapy alone (Group B). Group A received MEL therapy twice weekly, with bi-weekly intradermal PRP. Group B received MEL therapy twice weekly for a 4-month maximum, or until complete repigmentation. The study found that PRP combined with MEL therapy did not benefit the treatment of localised vitiligo.52 PRP has also been utilised in the treatment of melasma. In a split-face trial, the side treated with PRP showed significant improvement after treatment for 6 weeks.53 Other studies tested PRP with hydroquinone or tranexamic acid for melasma, and showed an improvement when PRP was added as an adjuvant.54
OTHER (n=47)
Primarily documented in the realm of regenerative medicine, PRP formulations have been utilised in diverse medical disciplines, including gynaecology and urology.55,56 Notably, PRP’s wound healing attributes can offer significant benefits following a Caesarean section procedure. In a comprehensive study exploring the potential gynaecologic utility of PRP, a cohort of 200 females undergoing Caesarean section were randomly assigned to two groups.55,56 One group received PRP injections after the surgery, and the control group received standard care. The PRP group demonstrated a greater reduction in the REEDA (redness, oedema, ecchymosis, discharge, and approximation) score on Days 1 and 7 after surgery, and continued to show greater improvement until 6 months. The Vancouver classification and the VAS were also significantly lower in the PRP group.
PRP has also been investigated as a possible treatment for erectile dysfunction (ED). In 2021, the first double-blind, randomised, placebo-controlled clinical trial assessing the use of PRP for ED treatment was performed.57 Poulious et al. randomly assigned 60 males with ED to either 10 mL of PRP injections or 10 mL of normal saline injections in two sessions, with a 1-month interval in between. At 6 months, the minimally clinically important difference was achieved by 69% of patients in the PRP group, compared to 27% in the placebo group (p<0.001). Between the two groups, the risk difference was 42% (95% confidence interval: 18–66).
CONCLUSION
As summarised, PRP has been explored as a potential therapeutic intervention in a variety of medical fields, yielding variable results. Its utilisation in musculoskeletal, maxillofacial surgery, wound healing, dermatology, and other fields such as gynaecology and urology have demonstrated potential benefits in certain cases, while in others, no significant distinctions have been observed compared with alternative treatments or placebo.
The existing body of literature faces substantial limitations. These encompass issues such as small sample sizes, short follow-up durations, and lack of standard protocols for PRP preparation, which collectively hinder the comparability of PRP’s efficacy among different studies. The variability in measured end outcomes, as well as a great heterogeneity in PRP preparation and administration strategies, adds to the complexity. For instance, the platelet counts in PRP can vary greatly depending on the chosen preparation technique, potentially influencing study outcomes.1 Unfortunately, the current literature often fails to adequately document these nuanced distinctions, impeding the ability to conduct accurate cross-study comparisons.
This situation has led to the proposal of numerous classification systems, with two prominent classification systems, the Scientific and Standardization Committee (SSC) of the International Society on Thrombosis and Haemostasis, Inc. (ISTH) and Khan et al., aiming to address the challenges of inconsistent reporting methods and lack of understanding of PRP components influencing clinical outcomes.12 However, many studies still fail to report aspects of PRP preparation, such as growth factor levels, hampering classification efforts.
Moreover, while the global market for PRP was valued at 627.9 million USD in 2022,59 a robust characterisation and understanding of PRP’s constituents remain an ongoing debate within the scientific community. Blinded RCTs with objective outcome measurements are necessary to fully assess the efficacy of PRP. Further research into different preparation techniques, the best PRP administration strategies, and intervals between injections is vital.
A study analysing trends in PRP usage and costs of injections in orthopaedic surgery demonstrated usage significantly increased between 2010–2019.12,58 The average cost of a PRP injection clustered around 1,000 USD.58 In 2022, the global market for PRP was valued at 627.9 million USD, and the expected compound annual growth rate from 2023–2030 is 15.1%.9 Medicare offers reimbursement for PRP injections when they are performed for patients with chronic, non-healing diabetic, pressure, and/or venous wounds enrolled in an approved clinical research study.59 A study analysing Medicare billing for PRP reports annual charges to Medicare increased from 500,000 USD in 2010 to more than 2 million USD in 2014, a 400% increase.60 These studies demonstrate the growing popularity of PRP in the medical settings. As such, an increase in research to further establish its efficacy, safety, and the best administration and assessment strategies is necessary.
As a growing interest in PRP is evident across diverse medical fields, the implications of the authors’ findings are profound. PRP, while showcasing potential benefits in musculoskeletal, maxillofacial surgery, dermatology, and other specialisations, has yet to prove its unequivocal superiority in all scenarios. Looking ahead, as the field of regenerative medicine continues to evolve, the onus is on the scientific community to devise standardised protocols for PRP preparation, classification, and reporting. Achieving this uniformity will allow for a more clear insight into PRP’s therapeutic viability, and spotlight clinical scenarios where it can be most beneficial. The roadmap for future research should prioritise surmounting these challenges, refining PRP formulations, and discerning the most efficacious PRP components for diverse clinical applications. Embracing these strategic shifts will be pivotal in realising the full therapeutic potential of PRP.






