Abstract
Mycosis fungoides is the most common form of cutaneous T cell lymphoma. In this article, the authors describe a case of a 53-year-old female who presented with multiple pruritic, slightly erythematous and hyperpigmented, variably shaped macules, and patches and plaques, with scaling on the bilateral hands and feet. The lesions resembled various benign inflammatory dermatoses, such as contact dermatitis and superficial dermatophytosis, and were treated as such until hyperpigmented, variably shaped macules and patches appeared on the legs and with a bathing trunk distribution. Histopathologic and immunohistochemical findings were consistent with early mycosis fungoides. The patient has improved with full body narrowband ultraviolet B phototherapy, combined with a psoralen ultraviolet A soak for the hands and feet. This case highlights the importance for physicians to recognise that mycosis fungoides may occur with different presentations, and may not present in its classic form. The patient’s quality of life may be improved with early diagnosis.
Key Points
1. Diagnosing early mycosis fungoides relies on clinicopathologic correlation. Multiple skin biopsies examining histology, immunohistochemistry, and T cell receptor monoclonal rearrangement, if feasible, alongside thorough correlation of clinical and pathological data, are crucial for accurate detection of signs of mycosis fungoides.
2. Detecting mycosis fungoides necessitates a robust clinical suspicion of cutaneous T cell lymphoma. This is especially crucial when dealing with chronic, unyielding inflammatory skin disorders that do not respond to treatment.
3. In cases of early-stage disease, it is prudent to give due consideration to the implementation of safer treatments like topical medications and phototherapy. These options can contribute significantly to the management and care process.
INTRODUCTION
Mycosis fungoides is the most common form of cutaneous T cell lymphoma, which is a heterogenous group of non-Hodgkin lymphomas.1 Classic early mycosis fungoides typically begins as persistent and/or slowly progressive scaly pink or red patches on the hands, chest, abdomen, upper thighs, or buttocks.2,3 The lesions are often pruritic and may cause impairment of the quality of life.3 On histology, superficial lymphoid infiltrate are present with characteristic epidermotropism, without spongiosis and lymphoid atypia.4 Immunophenotypically, the cells are characterised by epidermotropic peripheral T lymphocytes, whose phenotype is (cluster of differentiation) CD2+, CD3+, CD4+, CD5+, CD8-, CD45RO+, CD20+, and CD30-, with few exceptions.5 The case of a 53-year-old Filipino female, who developed the classic variant of early mycosis fungoides after a few months with CD8+ mycosis palmaris et plantaris, is described in this report.
CASE REPORT
A 53-year-old Filipino female presented with a 10-month history of extremely pruritic patches on the plantar aspect of both feet, which later involved the palmar aspect of both hands. They were seen and managed by several dermatologists, and were treated as having xerosis cutis, dermatophytosis, and hand and feet dermatitis. Despite trials of various medications, such as topical and oral steroids, topical antifungal medications, and emollients, and in absence of any clear trigger, the lesions did not resolve. However, no initial histopathologic examination was performed.
The lack of improvement of symptoms; the evolution into erythematous scaly plaques; and the appearance of non-pruritic, non-painful brownish macules and patches over the bilateral legs, which progressed to involve the lower abdomen and buttocks in a bathing trunk distribution 6 months later, prompted consultation at the authors’ institution. Review of systems revealed absence of lymphadenopathy, significant weight loss or gain, and presence of hair thinning but without alopecia. The past medical, family medical, and personal and social history were unremarkable. Cutaneous examination revealed multiple, well- and ill-defined, slightly erythematous and hyperpigmented, variably shaped macules, patches and plaques, with scaling on the bilateral hands and feet, legs and bathing trunk distribution (Figure 1). No lymphadenopathies were observed on palpation.
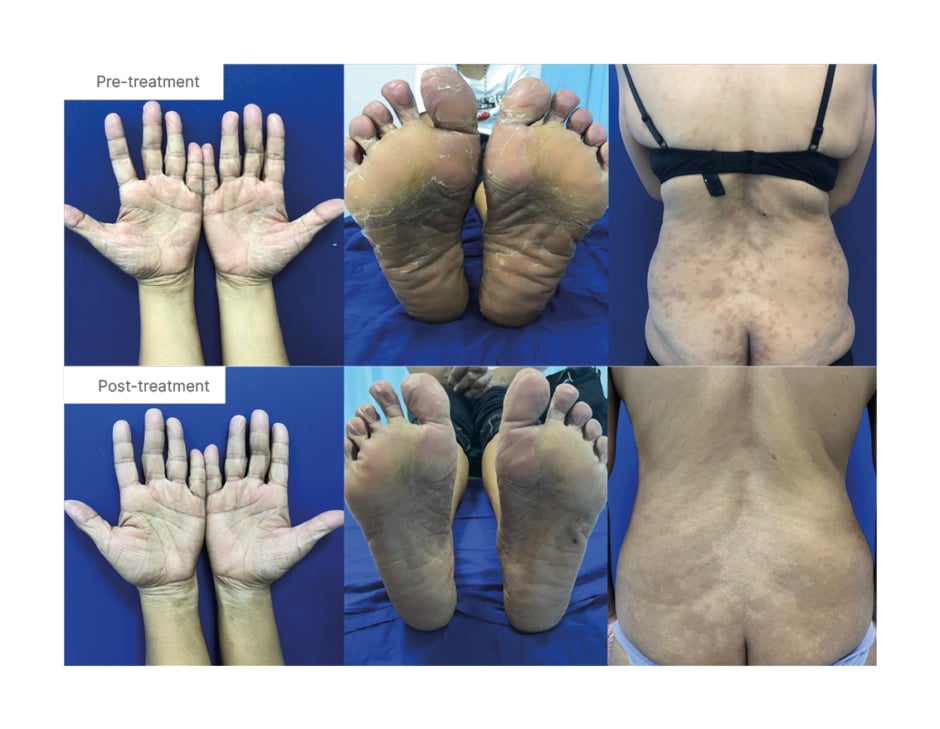
Figure 1: Mycosis fungoides palmaris et plantaris progressing to complete classic mycosis fungoides.
Clinical images of hands, feet, and back at diagnosis, and after 18 sessions of full body NB-UVB phototherapy, PUVA soak of the hands and feet, and 2 months of topical clobetasol.
NB-UVB: narrowband-ultraviolet B; PUVA: psoralen ultraviolet A.
KOH test and syphilis test were requested; both revealed negative results. A biopsy was performed on the palm and the lower back, which showed orthokeratosis with parakeratosis, epidermal hyperplasia with scant spongiosis, superficial perivascular infiltrates of lymphocytes, few eosinophils, and focal lichenoid interface dermatitis/basal vacuolar change. Atypical lymphocytes infiltrated up into the epithelial layers (epidermotropism) in single units or small clusters. Tissue cells showed positive staining for CD3, CD4, and CD8. The ratio of CD4- to CD8-positive cells was about 3:1. CD7, another marker for T cells, appeared to be intact, but with downregulation compared with CD4 (Figure 2). The cells of interest were negative for CD20 and CD30. Blood count demonstrated mild anaemia and lymphocytopenia. On peripheral blood smear, slightly hypochromic erythrocytes with anisopoikilocytosis and adequate platelet count were observed. No abnormal white blood cells were seen. PET–CT scan results showed low-grade metabolic activity in multiple nodes, which were regarded as reactive or inflammatory without hypermetabolic gross soft tissue masses or evident cutaneous thickening in the extremities and body wall. T cell receptor clonal analysis was not conducted in this case because the morphological evaluation with immunostaining profiling was diagnostic.
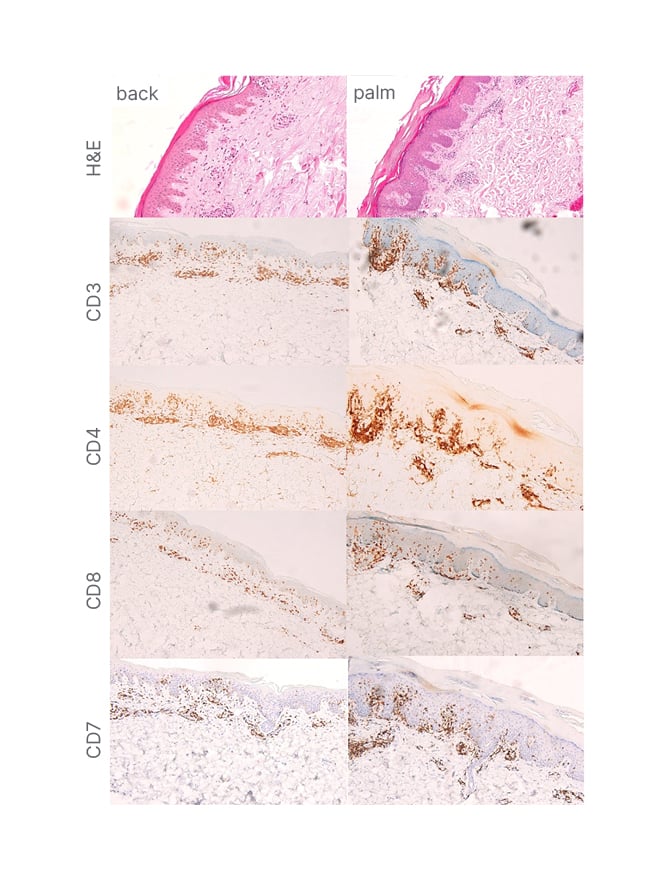
Figure 2: Histopathology and immunostaining profiles of mycosis fungoides palmaris et plantaris progressing to full-blown classic mycosis fungoides.
A) An infiltrate of the atypical lymphocytes in the upper dermis (H&E, 20×1). B–D) Positive CD3 and CD4 atypical lymphocytes. D and E) Reduced CD8 and CD7 expression in atypical lymphocytes.
CD: cluster of differentiation; H&E: haematoxylin and eosin.
Full body narrowband-ultraviolet B (NB-UVB) phototherapy was initiated at 300 mJ/cm2, and increased by 10% increments per session, 2–3 times a week. Eventually, the authors decided to combine treatment with a psoralen ultraviolet A (PUVA) soak for the hands and feet, starting at 0.5 J/cm2, and increasing by 0.5 J per session. In addition, topical clobetasol lotion 0.05% was applied twice daily continuously for 3 months, and loratadine 10 mg/tab was given as needed for the pruritus once a day. Marked decrease in the number of hyperpigmented macules and patches was noted after 18 sessions with NB-UVB phototherapy. With use of the PUVA soak, >90% of the lesions on the palms and soles have resolved. However, after 18 sessions of the PUVA soak, the patient experienced increased pruritus in these areas, which prompted the authors to adapt the panel NB-UVB for the hands and feet (Figure 1). The patient is currently undergoing maintenance phase dosing for the body, with almost complete resolution of lesions and residual hyperpigmented patches seen, as well as on the acral areas with almost complete clearance. The authors plan to further decrease the frequency of visits to once a week to complete 6 months.
DISCUSSION
Mycosis fungoides is the most common type of cutaneous T cell lymphomas, which represents almost 50% of all cases.6 It is characterised by malignant proliferation of CD4+ T cells, with epidermotropism in the skin. The disease is more commonly seen in individuals in their mid-50s, increasing its incidence significantly with age, and demonstrating a four-fold in incidence in patients over 70 years.7 The diagnostic algorithm for the diagnosis of early mycosis is based on a point-based algorithm proposed by the International Society for Cutaneous Lymphoma (ISCL) and the European Organization of Research and Treatment of Cancer (EORTC). The diagnosis is made when a total of four points or more is determined.4
Among these criteria, clinical presentation is one of the significant factors in the diagnosis of early mycosis fungoides. About 70–75% of patients with mycosis fungoides present the classic form of the disease, characterised by commonly pruritic, well-demarcated, erythematous patches and/or plaques with variability in the size, shape, and colour, and occasional poikiloderma. The lesions are usually large in size, around >5 cm, and have predilection to non-sun-exposed areas, such as the buttock, flanks, inner thighs, and inner arms. The fixed character of the lesions or the waxing and waning of the skin lesions over months, or even years, should incline the clinician on a diagnosis of mycosis fungoides.4
Lebas et al.8 described the mimicker and variants of mycosis fungoides, including hyperkeratotic, hypopigmented mycosis fungoides associated with follicular mucinosis, granulomatous slack skin, pagetoid reticulosis, interstitial mycosis fungoides, poikilodermatous, anetodermic, and invisible types. Interestingly, the authors’ case initially presented as mycosis fungoides palmaris et plantaris before it progressed into a full-blown, early disease. This exceedingly rare variant is more predominant in males. The lesions occur in the palms and soles, and can present variably as annular, erythematous to hyperpigmented patches and plaques,9 tumours, pustules, verrucous changes, ulceration, and nail dystrophy.10,11 It has an indolent course, remaining confined to the area of initial involvement with limited spread, which is paradoxical in the authors’ case, wherein lesions progressed to the classic type of mycosis fungoides. Dermatophyte infections, eczema, palmoplantar psoriasis, or secondary syphilis should always be ruled out in the diagnosis.9 Histopathologically, mycosis fungoides palmaris et plantaris may have spongiotic dermatitis, making a correct diagnosis difficult in some cases. Thus, immunophenotyping and T cell clonality must be performed to confirm the diagnosis. CD8+ aggressive epidermotropic T cell lymphoma has been associated with metastatic involvement of distal anatomic sites and poor prognosis.12 The authors’ patient presented with a rare case of CD8+ cytotoxic T cell lymphoma, without peripheral blood involvement, and good response to treatment.
Early mycosis fungoides has an indolent course, and thus, safer treatment options should always be considered. In patients with patch/plaque stage mycosis fungoides and mycosis fungoides palmaris et plantaris, the primary goal is to improve symptoms and quality of life while avoiding treatment-related toxicity because of its excellent prognosis.7 This involved either expectant management through watchful waiting, or the utilisation of skin-directed therapies.7,13 Current skin-directed treatment options for early mycosis fungoides include topical corticosteroids, topical chemotherapy (i.e., nitrogen mustard), topical retinoids (i.e., bexarotene or tazarotene), topical imiquimoid,14 phototherapy, local radiation therapy, and total skin electron irradiation for patients with severe skin symptoms.15 Phototherapy is an important treatment modality that may be used alone, or in conjunction with topical therapies. The reported complete response rates, defined as >90–95% clearance, for early stage mycosis fungoides (Stage IA–IIA) for NB-UVB ranged from 54–90%, while the complete response rate was reported as 65–85% PUVA.16 In a systematic review and meta-analysis by Phan et al.17 comparing the efficacy of NB-UVB with PUVA, they concluded that NB-UVB, due to its safety profile, is a viable alternative to PUVA for treatment of early-stage mycosis fungoides.
CONCLUSION
Mycosis fungoides is an exceedingly uncommon skin condition with a variety of clinical manifestations, making its diagnosis still challenging without the adjunct of immunohistochemical staining and T cell receptor gene rearrangement. Thus, clinicopathologic correlation is the best way of diagnosis. The authors highlighted the importance of high clinical suspicion for cutaneous T cell lymphoma in patients with chronic unremitting inflammatory dermatoses that are recalcitrant to treatment. Phototherapy alone, or in conjunction with topical therapies, is both a viable and safe treatment option for the management of early-stage mycosis fungoides.






