Abstract
Ungual psoriasis and onychomycosis are common nail diseases. Despite their different aetiology and course, surprisingly they have much in common both clinically and histopathologically, rendering their distinction often very challenging. Because their treatments are fundamentally different, anti-inflammatory–immunosuppressive for psoriasis and anti-infective for onychomycoses, an exact diagnosis is crucial for their management. Psoriasis is the dermatosis with the most frequent nail involvement. Pits, ivory-coloured spots, salmon or oil spots, subungual hyperkeratosis, onycholysis, and splinter haemorrhages are the most common nail signs. Onychomycoses are thought to be the most frequent nail diseases. This statement is disputed for toenails, for which orthopaedic abnormalities are said to be even more frequent and mimic fungal nail infections.
INTRODUCTION
Nail disorders account for approximately 10–15% of the workload of dermatologists. Patients request, and deserve, an accurate diagnosis and treatment.1,2 This can be difficult when the most frequent onychopathies such as nail psoriasis, onychomycoses,3,4 and nail alterations of the asymmetric gait nail unit syndrome are clinically very similar.5 They all have a severe impact on a patient’s quality of life.6
UNGUAL PSORIASIS
Psoriasis is the dermatosis that most frequently affects the nails.2 Approximately half of psoriasis patients have nail alterations at any time, but 80–90% will experience nail changes at least once during their lifetime.2,7 Nail psoriasis is frequently associated with arthritis, particularly of the distal interphalangeal joints. The nails are even more often involved in psoriatic arthritis.8 Fingernails are more frequently affected than toenails.2,7 In contrast to cutaneous psoriasis, there is no association of nail psoriasis and psoriatic arthritis with HLA-C0602.7 Nail psoriasis is also frequently associated with enthesitis, an inflammation of tendon and ligament insertions. This is interpreted as being a Köbner phenomenon rather than an autoimmune disease.9
Psoriasis causes both specific and less characteristic nail alterations (Figure 1A).10,11 Pits are the most frequent alterations of matrix psoriasis.2 They develop from minute psoriatic foci in the apical matrix resulting in circumscribed parakeratotic mounds that break out and leave a small depression in the nail plate surface.12 However, some parakeratotic foci often remain and are seen as small whitish to ivory-coloured spots. The pits and spots are of relatively regular depth and size with random distribution; however, they are sometimes arranged in horizontal or longitudinal lines. More than 10 pits per nail or a total of more than 60 pits and spots are generally seen as confirming the diagnosis of nail psoriasis (Figure 1A).2 Psoriatic lesions in the distal matrix may be seen as red spots in the lunula and those in the mid-matrix may cause psoriatic leuconychia. The most common signs of nailbed involvement are subungual hyperkeratosis and oil or salmon spots. When the latter grow to the hyponychium they result in onycholysis, which is typically bordered proximally by a reddish-brown band reflecting an active psoriatic lesion.2,12 Small, thin, longitudinally arranged dark lines are splinter haemorrhages and correspond to microthromboses of the longitudinally arranged nailbed capillaries;2 thus, they are comparable to Auspitz’s phenomenon.
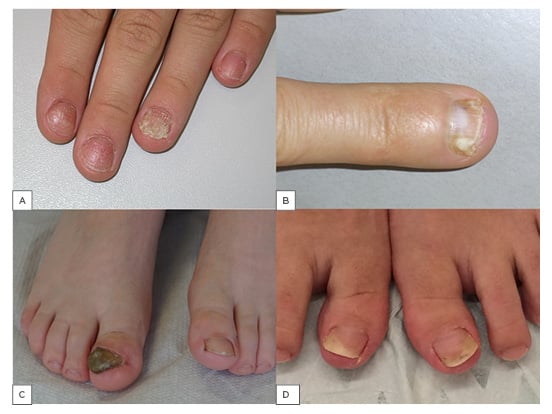
Figure 1:
A) Nail psoriasis; B) deep type of superficial white onychomycosis; C) massive nail thickening, discolouration, onycholysis, distal bulge formation, and lateral deviation in right-sided congenital malalignment of the big toenail; D) AGNUS of both big toenails: inward rotation of the toes, slight hallux erectus, and onycholysis of the distal lateral nail portion.
Involvement of the proximal nailfold leads to psoriatic paronychia with spontaneous loss of the cuticle. Affection of the entire nail apparatus causes complete nail destruction. Depending on the extent and severity of the involvement of the different parts of the nail, extremely variable intra- and interindividual clinical patterns can be seen. Finger and toenail involvement often present different clinical aspects. The distal phalanx is swollen in psoriatic arthritis and the joint may be stiffened in a slightly bent position. Psoriatic pachydermoperiostosis is almost exclusively observed on big toes with minor or no nail changes.13
All three forms of pustular psoriasis affect the nails.2 Small yellowish spots are seen under the nail in generalised pustular psoriasis of von Zumbusch, whereas larger lakes of pus can be observed under the nail together with larger surface defects called elkonyxis in palmar plantar pustular psoriasis.2,7 Acrodermatitis continua suppurativa of Hallopeau is an excessively recalcitrant disease, mostly isolated on the tip of a digit. This may turn red, inflamed, develop small to medium-sized pustules that destroy the nail, and finally result in a rounded naked digit tip without a nail.13 Concomitant psoriasis lesions elsewhere on the skin may develop but this is rather rare. As seen in generalised pustular psoriasis, an IL-36 receptor antagonist defect was found in acrodermatitis continua suppurativa.14 Because of its frequent monodigital involvement, acrodermatitis continua suppurativa often remains undiagnosed for years. An important differential diagnosis is nail involvement in reactive arthritis.13 Additionally, nail psoriasis is also observed in children. The diagnosis is often missed because paediatricians commonly do not think of psoriasis in this age group.15
Approximately 5% of cases are isolated nail psoriasis without skin lesions.2,4 A diagnostic adjunct to the clinical diagnosis and to avoid an invasive biopsy is the histopathological examination of nail clippings with as much of the subungual hyperkeratosis as possible.11,12,16-18 As nail psoriasis has a serious negative impact on the quality of life, an early and exact diagnosis is warranted. Chronic trauma and professional stress to the digit aggravate nail psoriasis. The common course is chronic or chronic recurrent. This waxing and waning of nail lesions often helps to distinguish it from onychomycosis.7,13 For scientific reasons and therapeutic studies, nail psoriasis grading systems were established to reproducibly determine the extent and severity of ungual psoriasis. The Nail Psoriasis Severity Index (NAPSI) is used most frequently although several other grading systems were created that also cover other aspects.
ONYCHOMYCOSES
Fungal infections of the nail unit are commonly designated as onychomycoses. They are said to be the most frequent nail diseases constituting 40–50% of all nail disorders. They are distinguished by their responsible pathogens and the route of infection determining the nail structures primarily involved.19,20 This classification is particularly important for onychomycosis treatment and prognosis.
The most common pathogens of onychomycoses are dermatophytes, which contain specific enzymes capable of degrading keratin. Trichophyton rubrum, followed by T. interdigitale (mentagrophytes) are the leading pathogens; T. soudanense, T. violaceum, T. tonsurans, and Microsporum spp. rarely cause nail infections.21 Some Candida species contain acid peptidases that can digest nail keratin, although they are more commonly found in chronic paronychia of fingers. C. albicans and C. parapsilosis are the leading yeasts, but C. glabrata, C. tropicalis, and C. krusei are uncommon. Nondermatophyte moulds are now also accepted as being primary nail pathogens, particularly Scopulariopsis brevicaulis22 and Fusarium spp., with the latter presenting as new emerging nail pathogens.23 There are differences in the spectrum of nail pathogens depending on geography, climate, and common habits;24,25 however, clinical distinction of the different pathogens is usually not possible.19 Yeasts and nondermatophyte moulds are comparatively more frequent in psoriatic nails.26,27
Estimates of the prevalence of onychomycoses differ.24 Between 3% and 8% of the population are said to have fungal nail infections; however, in some professional groups, the prevalence was 8–40%. Of patients with tinea pedum, 20–30% have onychomycoses. Men appear to be affected more frequently than women and the frequency increases steadily with age.28 The susceptibility to develop an onychomycosis is an autosomal dominant trait evidenced by the frequent vertical spread within affected families.29 Those with psoriasis will experience fungal nail infections more frequently, making the differential diagnosis difficult or impossible.2,4,12 Toenails growing only one-third of the rate of fingernails are 7–10 times more frequently infected. Mixed infections make up for 5% of all onychomycoses,19,20,30 and immunosuppressed individuals are prone to rare fungal species that are usually difficult to treat.19
The differentiation of onychomycoses according to the route of invasion is important in clinical practice because it also explains the severity and chances of a successful therapy.31 By far the most common type is distal lateral subungual onychomycosis (DLSO).1 From the infected skin of the tip of the digit and lateral nail folds, the fungus grows into the hyponychium and then invades the nailbed. This reacts with a mild distal hyperkeratosis that extends proximally and thickens eventually raising the nail. The overlying nail plate covers the infection and only later gets invaded, which is seen by the loss of transparency and fragility of the plate (Figure 1B).19,31 Histopathology of nail clippings with subungual hyperkeratosis demonstrates fungi in the keratin and undersurface of the nail. It shows that the nail is not the primary target but rather a barrier for the fungus.12 The further course of the infection is characterised by slow invasion into the direction of the matrix; however, this may remain stable for months or years in many cases. Dermatoscopy often shows a fringed proximal border compared to an aurora borealis.32 Another feature not infrequently seen in toenails is the development of a yellow spike pointing proximally, extremely rich in thick-walled fungi including both short filaments and spores, and therefore also called dermatophytoma. It is very recalcitrant and usually requires mechanical debridement for treatment. After years or decades, this DLSO can involve the entire nail and destroy it. White superficial onychomycosis (WSO) is divided into 3 subtypes. The classical form of WSO exhibits chalk-white spots with a lustreless surface on toenails and arises due to a particular growth pattern of T. mentagrophytes. Another form is seen in immunocompromised patients, primarily observed on fingernails, arises due to T. rubrum, and has a shiny surface. The third form is the ‘deep’ WSO, which develops when the classical form of WSO extends under the proximal nailfold and, because of this occlusion, can invade into the nail plate (Figure 1B). Proximal subungual white onychomycosis develops when a pathogenic fungus breaks the barrier of the cuticle and grows along the eponychium in a proximal direction until it reaches the matrix from where it is both included into the growing nail plate and actively invades distally towards the nailbed. A rare form caused almost exclusively either by T. soudanense or T. violaceum is endonyx onychomycosis, which histopathologically shows fungal organisms in the middle layer of the nail plate but without nailbed involvement.
albicans has enzymes capable of splitting up keratin. Particularly in hot climates, an infection similar to DLSO is observed whereas in temperate climates, paronychia may develop. Proximal subungual white onychomycosis caused by Candida spp. is occasionally observed in neonates. Nondermatophytes are increasingly found in onychomycoses;5 however, their aetiopathogenetic role is not always clear. All forms of onychomycosis can ultimately develop into total dystrophic onychomycoses, in which the nail is destroyed and substituted by keratotic debris. A primary total dystrophic onychomycosis is characteristic for chronic mucocutaneous candidiasis.33
DIAGNOSIS OF FUNGAL NAIL INFECTIONS
Although onychomycoses are often diagnosed on clinical grounds alone, this should not be the standard because treatment is always long, tedious, and potentially associated with serious side effects.34 The most common examinations are direct microscopy of subungual keratotic material after clearing with potassium hydroxide plus mycological cultures. Direct microscopy is rapid, easy, and inexpensive, but often nonspecific. Although capable of identifying the fungus, cultures take 4–6 weeks, give false-negative results in 30–50% of cases, and cannot distinguish between a true invasive onychomycosis and colonisation. Histopathology of nail clippings only takes 1–3 days, is twice as sensitive as cultures, insensitive to contamination, allows the differentiation between infection and contamination to be made, and gives permanent preparations. It does not, however, permit species identification.35,36 For superficial white onychomycosis, a thin slice from the nail surface may be taken with a No. 15 scalpel blade and for proximal subungual white onychomycosis, a disc of nail plate may be punched out and then divided into halves for culture and histopathology. Nail clipping histopathology also allows psoriasis and onychomycoses to be differentiated by their different neutrophil and parakeratosis distribution.37,38 Immunohistochemistry should theoretically allow species identification in situ, but there were, until now, no reliable antibodies on the market. Similar problems exist for in situ hybridisation. New diagnostic techniques include PCR and matrix assisted laser desorption ionisation – time of flight (MALDI-TOF) mass spectroscopy.39 Both are expensive, require specialised laboratories, and cannot differentiate between true infection and contamination.
DIFFERENTIAL DIAGNOSES
The most important differential diagnosis of nail psoriasis is onychomycosis and vice versa.40 They have many clinical and histopathological features in common, though to a variable degree (Figure 1A and 1B) (Table 1). Onychoscopy may help in the differential diagnosis.41 Compared to onychomycosis, a thinner nail plate, structural bone changes, and a higher power Doppler signal was found in nail psoriasis by ultrasonography.42 Confocal laser scanning microscopy and optical coherence microscopy may identify intraungual fungi.43,44 Recently, a genetic susceptibility to acquire onychomycosis in psoriasis was found with HLA-DR*08 and HLA-DR*01, likely increasing the susceptibility to fungal nail infection.45 The prevalence of onychomycosis in psoriasis patients is estimated to be between less than a quarter to one-third,46-52 but was found in 50% of patients in a recent study from Italy; however, yeasts were statistically significantly more frequent in the non-psoriatic control group.53 In a case-control study from Pakistan, nearly one-third of nail psoriasis patients had onychomycosis.54 It was assumed that the pathogenic fungus benefits from the damaged nail of psoriasis.55
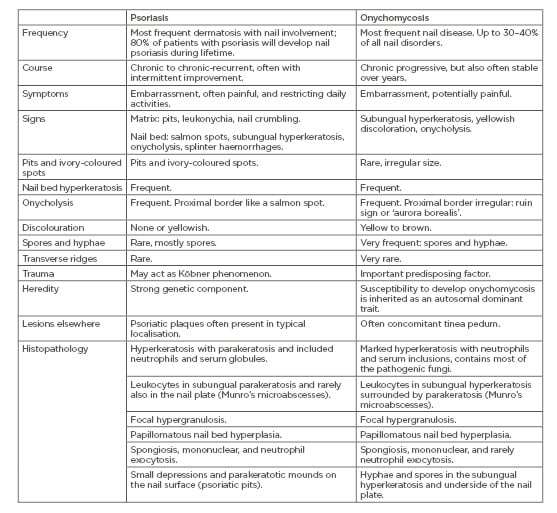
Table 1: Differential diagnostic features of nail psoriasis and onychomycoses.
Other very frequent differential diagnoses of toenail changes are caused by mechanical irritation such as friction from footwear, overlapping toes, or sports activities. The asymmetric gait nail unit syndrome is a characteristic condition seen in individuals with orthopaedic abnormalities that may begin in the vertebral column, continue over the hip to the knees, but is usually most obvious in the feet.5,56,57 This is associated with distal lateral or distal medial onycholysis in the innermost toes with a smooth border and without a reddish-brown margin (Figure 1D). It is commonly mistaken for a fungal nail infection. Histopathology and cultures are usually negative for pathogenic fungi. However, dystrophic nails are more often infected by fungi.58 Congenital malalignment of the big toenails is characterised by early onset lateral deviation of the nails, discolouration, oyster shell-like surface, and severe onycholysis (Figure 1C).59 Trachyonychia, or rough nails, could affect single nails or almost all nails, particularly in 20-nail dystrophy of childhood. It describes nail changes that may be idiopathic or due to atopic eczema, lichen planus, psoriasis, or, although rarely, some other dermatoses. Clinically, they typically cannot be distinguished, and their exact diagnosis requires the histopathological examination of a nail biopsy. Nail lichen planus is characterised by longitudinal ridging and splitting, as well as permanent scarring and pterygium formation. Nail eczema exhibits irregular pitting and transverse bulges and ridging, the proximal nail fold is often thickened, and the cuticle is lost. Rough nails may also be due to fungal infection, characteristically chronic mucocutaneous candidiasis. Chronic toenail conditions, particularly in the elderly and weaker individuals, may lead to onychogryphosis, which is defined by ram’s horn-like nails. These may show fungi in histopathology slides, but they are not the real cause of onychogryphosis. Pseudomonas aeruginosa often colonises predamaged nails causing a greenish discolouration. Onychotillomania and other habits occur both on finger and toenails and are often mistaken for a mycotic infection. Nail alterations in reactive arthritis may be almost indistinguishable from those of pustular psoriasis but are often more marked and the pustules have a brownish tinge due to frequent erythrocyte admixture. Palmar plantar lesions are seen as so-called keratoderma blenorrhagicum, and oral mucosal involvement is characteristic. Scabies may infest the nail unit, particularly in its crusted variant.60,61
Many nail diseases can be colonised or superinfected with fungi; it is then usually not possible to determine what was first. Psoriasis and onychomycosis may occur together.
As psoriasis treatment is usually immunodepressive, onychomycoses should be treated first.1
TAKE HOME MESSAGES
- Onychomycoses are the most frequent
nail diseases. - Psoriasis is the dermatosis with the most frequent nail involvement.
- Up to 80–90% of all individuals with psoriasis will develop nail lesions in their lifetime.
- Onychomycoses are the most frequent and resistant fungal skin infections.
- Onychomycosis treatment requires proof of the fungal aetiology, although this is not
always possible. - Nail psoriasis and onychomycoses have many signs and symptoms in common and may co-occur, sometimes rendering their differential diagnosis very difficult.







