Abstract
The psychosocial impact of alopecia on the quality of life of patients underscores the importance of dermatologist readiness to evaluate this common chief complaint. When evaluating a patient presenting with new-onset hair loss, the differential diagnosis may be broad, encompassing many subtypes of hair loss. Each type of scarring or non-scarring alopecia presents with its own unique aetiology, epidemiology, clinical presentation, trichoscopic findings, and laboratory studies. Further, accurate diagnosis is needed to determine appropriate therapeutic management. This review provides a systematic approach for dermatologists to use in order to accurately diagnose hair loss disorders, including clinical examination, laboratory evaluation, and specialised tests.
Key Points
1. Patients who experience hair loss commonly report embarrassment, changes to social and leisure activities, and a lower ability to engage in work and education. The psychosocial impact of hair loss on the quality of life of patients underscores the importance of dermatologist readiness to evaluate this common chief complaint.2. When evaluating a patient presenting with new-onset hair loss, the differential diagnosis may be broad. Each type of scarring or non-scarring alopecia presents with its own unique aetiology, epidemiology, trichoscopic findings, and potential management tools. This review provides a systematic approach for dermatologists to use to diagnose hair loss disorders, including clinical examination, laboratory evaluation, and specialised tests.
3. To successfully diagnose and treat patients with hair loss, evaluation begins with a thorough history and physical examination. Pertinent history includes evaluation of alopecia onset and duration, as well as a review of the patient’s medication, infection, and family history. Physical examination should be used to assess the overall hair density, and the pattern and distribution of hair loss.
INTRODUCTION
Hair thinning and hair shedding are complaints commonly encountered in clinical practice. Evaluating a patient with alopecia requires knowledge of the various aetiologies of hair loss, which may be bolstered by understanding the hair follicle cycle. The three main phases of the hair follicle cycle include: anagen or growth phase; catagen or involution phase; and telogen or resting phase, where the duration of anagen determines the hair length.1,2 This article aims to provide clinicians with a systematic approach to hair loss evaluation, including clinical examination, laboratory evaluation, and specialised tests (Figure 1). Clinical and trichoscopic characteristics of non-scarring (Table 1) and scarring alopecias (Table 2) are summarised. To successfully diagnosis and treat patients with hair loss, evaluation begins with a thorough history and physical examaniation.1-3
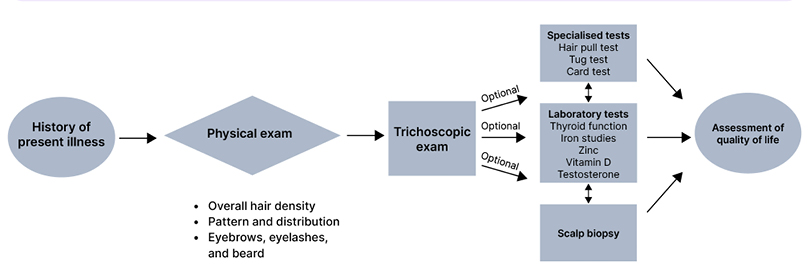
Figure 1: Approach to a patient of hair loss.
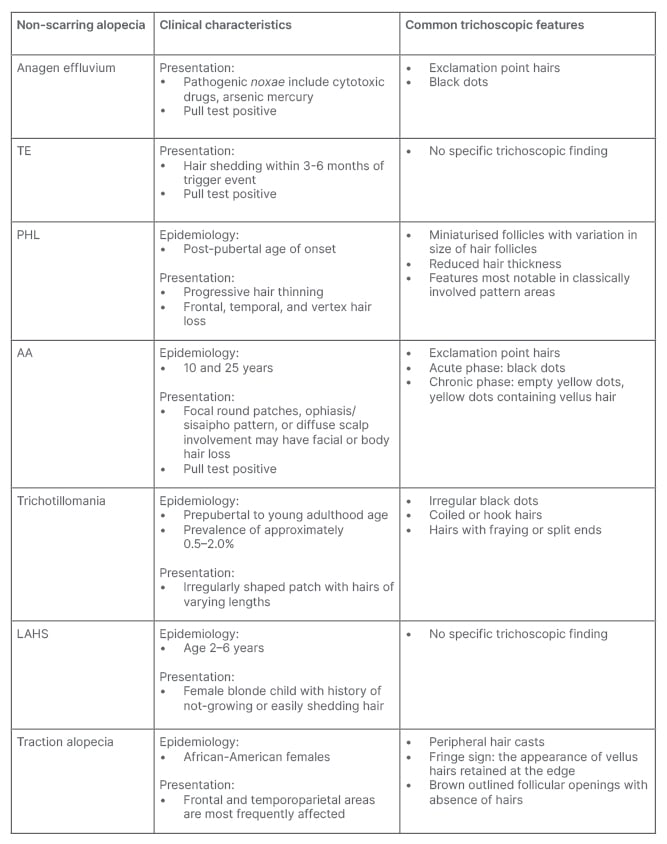
Table 1: Clinical characteristics and trichoscopic features of non-cicatricial alopecia.
AA: alopecia areata; LAHS: loose anagen hair syndrome; PHL: pattern hair loss; TE: telogen effluvium.
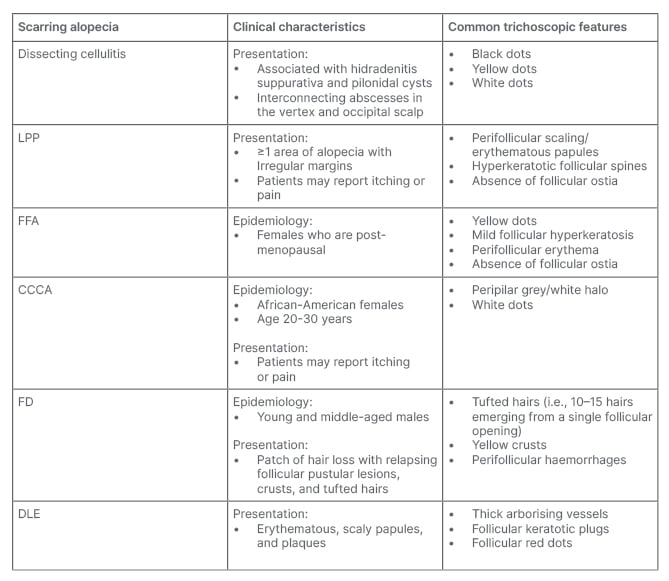
Table 2: Clinical characteristics and trichoscopic features of cicatricial alopecia.
CCCA: central centrifugal alopecia; DLE: discoid lupus erythematous; FD: folliculitis decalvans; FFA: frontal fibrosing alopecia; LLP: lichen planopilaris.
PHYSICAL EXAM: GLOBAL ASSESSMENT
Overall Hair Density
When inspecting the scalp, it is important to assess the overall density of hair. To evaluate hair density, isolate serial sagittal parts of the hair using your finger or a cotton swab. Start at the frontal hairline to the crown and then move to the occiput and then to the crown. A visible scalp indicates at least a 50% reduction in hair density compared to normal.2 Hair density may help to differentiate between telogen effluvium (TE) and anagen effluvium (AE). In TE, approximately 20–50% of hair is lost, whereas 80–90% of hair is lost in AE.4
Pattern and Distribution of Hair Loss
Physical examination should be used to determine the pattern and distribution of hair loss. Notable patterns of hair loss include diffuse, focal, patchy, or complete alopecia. In patients presenting with diffuse hair loss, pertinent history may include increased hair shedding or hair thinning (Figure 2). Gradual and progressive hair thinning is classically reported in patterned hair loss (PHL). Conversely, abrupt hair loss is more typical of TE, alopecia areata (AA), and AE. TE typically occurs 3–6 months after a triggering event, such as systemic disease, pregnancy, psychosocial stress, infection (e.g., COVID-19) or change of medication.2,3 If a patient presents with more localised hair loss, this is suggestive of early stages of PHL, frontal fibrosing alopecia (FFA), central centrifugal cicatricial alopecia (CCCA), AA, trichotillomania, and tinea capitis. The distribution of these patches may be frontal, crown, or random. Frontal predominance is highly associated with male PHL and FFA. A crown distribution is associated with female PHL and CCCA. Randomly distributed hair loss can suggest AA; scarring alopecias, such as lichen planopilaris (LPP) or discoid lupus erythematosus; trichotillomania; or tinea capitis. Importantly, more than one pattern and distribution of hair loss can co-exist, which may complicate diagnosis and assessment.2,4
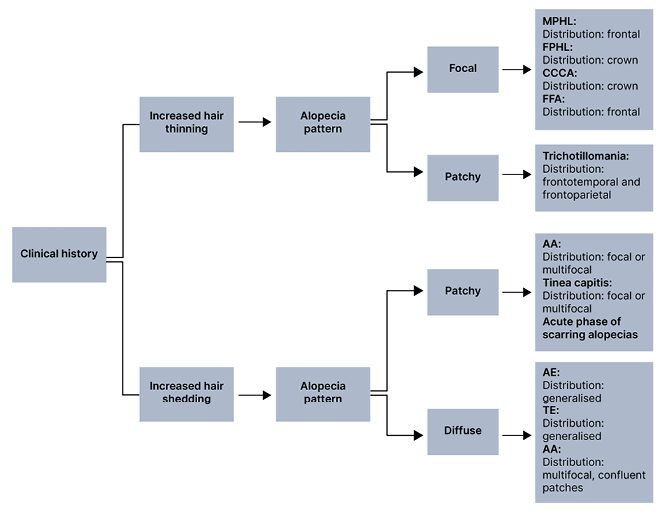
Figure 2: Pattern and distribution of hair loss.*
*More than one pattern and distribution of hair loss can co-exist.
AA: alopecia areata; AE: anagen effluvium; CCCA: central centrifugal cicatricial alopecia; FFA: frontal fibrosing alopecia; FPHL: female pattern hair loss; MPHL: male pattern hair loss; TE: telogen effluvium.
Additionally, hair shaft disorders with increased fragility are typically patchy but can also present with a generalised pattern. Notably, the pattern of AA is variable. At onset, patients with AA may report one or more round patches of alopecia; however, diffuse hair thinning and total alopecia can also be seen.2,4
Involvement of Eyebrows or Eyelashes or Facial and or Body Hair
In addition to the scalp, it is important to assess facial and body hair. AA, FFA, trichotillomania, tinea, and congenital hair shaft disorders may involve the eyebrows and eyelashes.3,5 AA can also involve the beard and facial hair, and generalised total body hair loss can be seen in AA universalis. Pubic region hair loss is a common area of trichotillomania involvement. Body and facial hair loss in AE are dependent on the chemotherapeutic agent, dose, and duration of treatment.3,6 Graham–Little–Piccardi–Lassueur syndrome, a subset of LPP, is associated with non-scarring hair loss of the axilla and groin. If eyebrows and eyelashes are not affected this may suggest PHL or TE.2
Secondary Changes on the Scalp
Generally, perifollicular or widespread secondary changes reflect inflammation of the scalp, which is more typical of infectious hair loss (e.g., tinea capitis) or scarring alopecia. Common secondary changes on the scalp include scales or flakes, erythema, pustules, and/or crusts. Pustules or crusts are common in folliculitis decalvans and tinea capitis. Additionally, folliculitis decalvans and LPP may present with perifollicular scaling. In advanced CCCA, patients can have perifollicular and interfollicular scales with perifollicular erythema.2, Widespread adherent scaling can be seen in tinea capitis, often resembling generalised dandruff.5 Erythema is seen in LPP, FFA, CCCA, folliculitis decalvans, and dissecting cellulitis.
HISTORY EVALUATION
Onset and Duration
Hair loss duration and age of onset provide important diagnostic clues. In acute TE, hair loss usually lasts for less than 6 months.1,3,4 Conversely, PHL and hair shaft disorders with hair fragility tend to be chronic with a longer duration.2 Additionally, hair disorders may have preferential onset during different stages of life. For children, the most common hair loss disorders are AA and tinea capitis.8 Traction alopecia can begin in childhood, but has the highest prevalence in young adult feamles.2 Trichotillomania typically presents in prepubertal or young adults.2, 7 In females, PHL and FFA peaks after menopause, though PHL may present any time after puberty.2 Further, it is important to enquire about history of hair regrowth in the affected area to differentiate between scarring versus non-scarring alopecia which may provide insight into prognosis.2
Medication History
To exclude drug-induced alopecia, a thorough medication history, with increased focus on medications initiated 3–6 months prior to the onset of hair loss should be reviewed. Drug-induced alopecia is typically diffuse and non-scarring.2 AE is sudden loss of up to 90% of hair following administration of antineoplastic medication or radiation.2 Medications commonly associated with drug-induced TE include psychiatric medications (e.g., mood stabilisers and antidepressants), anticoagulants (e.g., warfarin and enoxaparin), antithyroid, β-blockers, and retinoids.2,3 Cessation of long-term oestrogen-containing oral contraceptives has also been associated with TE (Table 3).2
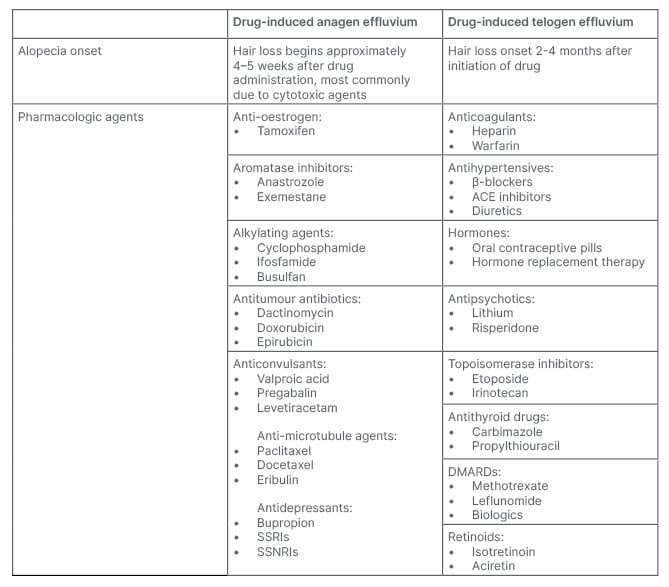
Table 3: Scalp and systemic infections that can cause alopecia.
AGA: androgenetic alopecia; TE: telogen effluvium.
Family History
Family history should be established in all patients, with special attention to hair loss disorders and other related conditions. AA frequently has a positive family history of other autoimmune disease, including vitiligo, pernicious anaemia, Hashimoto’s thyroiditis, and Type 1 diabetes.2,3 PHL, AA, and congenital hair shaft disorders typically have a positive family history of the same hair loss disorder.1,2 If PHL is suspected, it is important to ask about family history of frontal scalp hair thinning, as previous studies have reported that hereditary factors may predispose up to 80% of male PHL.4 If a child presents with alopecia, family history of childhood hair loss may suggest a hereditary shaft disorder.9
Infection History
Alopecia may be caused by scalp or systemic infections (Table 4). Bacterial or fungal infections that can enter the hair follicles or skin of the scalp and induce alopecia include tinea capitis, yeast (e.g., Candida and Malassezia), and Staphylococcus aureus. Clinically, tinea capitis may present with well-demarcated patchy alopecia with seborrheic-like scale.10 Pityriasis capitis secondary to Malassezia infection may result in chronic itch and inflammation which can exacerbate androgenetic alopecia (AGA) or induce TE.11 Systemic infections that can cause hair loss include secondary syphilis and COVID-19. Syphilitic alopecia can present in a diffuse form, resembling TE, or in a moth-eaten form resembling a variety of other focal alopecias (i.e., AA, trichotillomania, LPP, or tinea capitis).12,13
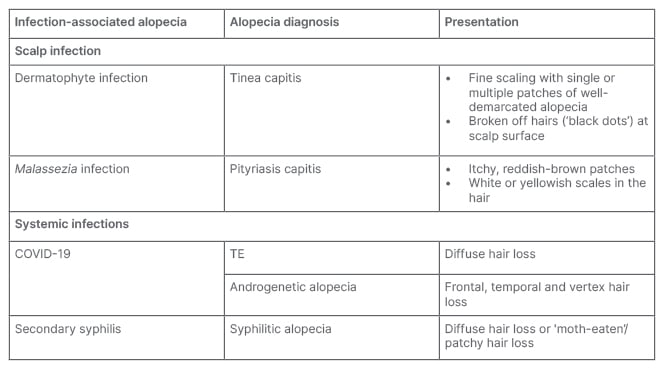
Table 4: Pharmacologic agents that may cause drug-induced alopecia.
ACE: angiotensin-converting-enzyme; DMARD: disease-modifying antirheumatic drug; SNRI: serotonin and noradrenaline reuptake inhibitors; SSRI: selective serotonin reuptake inhibitor.
During the COVID-19 pandemic, increasing numbers of patients were referred to dermatology with hair complaints.14 Therefore, dermatologists should be aware that COVID-19 infection can induce new-onset alopecia or exacerbate pre-existing hair loss.14 Mechanistically, new-onset diffuse, reversible hair loss (i.e., TE) can be triggered by viral mediated cytokine-storms.15 Additionally, AGA is a risk factor for severe infection due to androgen-mediated upregulation of the COVID-19 transmembrane receptor, which can exacerbate pre-existing patterned hair loss.16
Hair Care Practices
Physical and chemical factors have been shown to play a significant role in hair loss, thus current and past hair care practices should be evaluated.2 Repeated tension on hair, such as tight ponytails or buns, wearing turbans, and using hair extensions can cause traction alopecia.2 Excessive use of chemical hair treatment and excesses brushing can cause hair shaft disorders with increased fragility.2
Special Considerations for Females
Physiologic or iatrogenic changes in serum oestrogen should be considered in the evaluation of female patients with alopecia.
Pregnancy
Physiologic elevations of circulating oestrogen that occurs during pregnancy increases the number of hair follicles in the prolonged anagen phase, resulting in increased hair growth.17,18
Approximately 3–6 months after delivery, dramatic drops in hormone levels cause follicles to return to the telogen phase, resulting in excessive hair shedding known as postpartum TE.18,19 In patients without nutritional deficiencies or other systemic conditions, hair should return to baseline approximately 9–15 months after delivery.18, For patients reporting diffuse hair loss that persists for longer than 1–2 years postpartum, further investigation is warranted to evaluate for aetiologies of more chronic hair loss.18
Menopause
Decreased oestrogen secondary to menopause often results in changes in hair quality and structure, with hair becoming thinner and drier, and patients often report hair loss.20,21 Additionally, female PHL commonly presents in menopause, with weakening of the hair shaft and a wider part due to reduced hair density.21,22
Compared with females who are pre-menopausal, there is an increase in frequency of male pattern changes in females who are post-menopausal, with patients presenting with a receding hairline and thinning on the vertex scalp.22,23 FFA also presents in the peri-menopausal and post-menopausal state, but usually manifests as receding hair line localised to the frontal and frontotemporal region.1,9
Hormone Replacement Therapy
Hormone replacement therapy (HRT) is often prescribed to females who are post-menopausal to reduce vasomotor symptoms, such as hot flashes and vaginal atrophy.24 HRT most commonly uses combination therapy of oestrogen and progestin. It remains unclear if HRT worsens or improves hair loss, as oestrogens prolong the anagen phase in contrast to the androgenic effect of progestins.24 For patients taking HRT, clinicians should enquire their specific formulation as modifications in the proportion of androgenic progestins may improve alopecia.24
Special Considerations: Exogenous Testosterone
Testosterone disrupts the hair follicle cycle by shortening the anagen phase and reducing hair follicle diameter.22,23 For patients on exogenous testosterone, the increase in circulating testosterone may cause new onset male PHL.23 For transgender patients receiving hormone therapy, exogenous testosterone was found to be associated with male PHL and hirsutism.25
DIAGNOSTIC WORKUP
Laboratory Tests
In patients who present with new hair loss, it is common for preliminary laboratory tests to be obtained. Laboratory studies, such as complete blood counts, ferritin, thyroid, and vitamin D levels, can aid in understanding the aetiology of alopecia. However, the need for initial laboratory testing in the diagnostic workup of hair loss lacks consensus in the literature.26,27
Thyroid function tests
Alterations in the levels of thyroid hormones may function as a trigger TE. A study that included 1,232 patients with various types of alopecia found that patients aged 21–40 were most likely to have abnormal thyroid levels.28 Further, this study recommended that all patients presenting with new-onset alopecia be screened for thyroid abnormalities.28
Iron studies
The relationship between iron levels and alopecia is widely debated. Kantor et al.29 found that females under the age of 40 with female pattern hair loss and concurrent TE had lower levels of ferritin. However, Olsen et al.30 reported no significant difference in iron deficiency in hair loss patients compared with the control group. Furthermore, Sinclair31 found that iron supplementation did not improve alopecia.
Zinc
Patients with known zinc deficiency often experience alopecia, with regrowth occurring once supplementation is started.32,33 A systematic review found that 67% of patients with AA had decreased levels of zinc compared with those without hair loss.34 To date, however, there are no agreed upon recommendations for zinc supplementation in patients experiencing hair loss.34
Vitamin D
Studies have shown that females who experience female pattern hair loss are more likely to have low concentrations of serum vitamin D levels.35,36 Though the role of vitamin D in the hair growth cycle is known, the potential for vitamin D supplementation as treatment for hair loss is understudied.35
Others
When examining females with a history of infertility, hirsutism, or severe cystic acne, additional laboratory studies, such testosterone and prolactin levels should be considered.37 An English study found that up to 67% of females presenting with female pattern hair loss had polycystic ovary syndrome.37
Trichoscopy
Magnified assessment of hair characteristics can be performed with trichoscopy (Table 5). Trichoscopic evaluation should be used to assess the hair shaft, hair follicle openings (i.e., the follicular ostia), and perifollicular epidermis. Follicular ostia examination differentiates between scarring and non-scarring alopecia, where a lack of follicular ostia suggests scarring alopecia (e.g., FFA; Table 1). Scarring alopecia can also present with white dots, which indicate fibrosis, and may be seen in CCCA or inactive end-stage LPP (Table 2). Other differentiating follicular ostia trichoscopic features include black and yellow dots. Black dots indicate broken hairs at the level of the scalp, which commonly suggests AA, tinea capitis, and toxic-induced AE. Sebum or keratonic material on trichoscopic evaluation presents as yellow dots, which may suggest PHL or AA.2,7
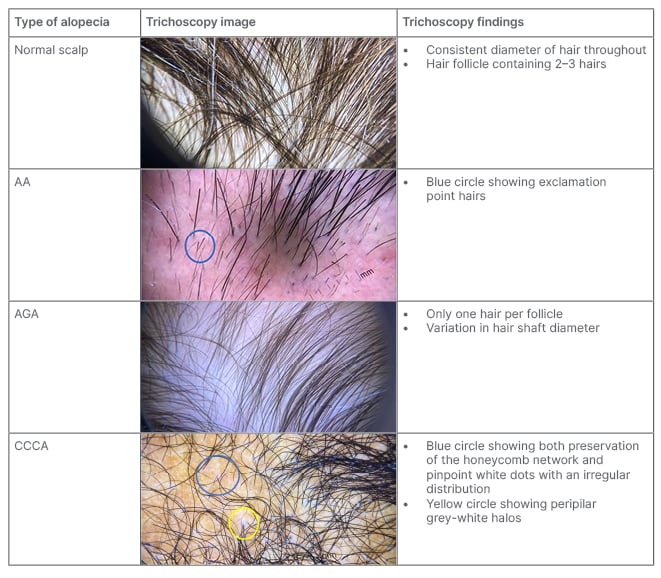
Table 5: Trichoscopic images of common hair loss disorders.
AA: alopecia areata; AGA: androgenetic alopecia; CCCA: central centrifugal cicatricial alopecia.
Hair shaft defects should be suspected in a patient who reports a change in hair texture, appearance, manageability, and/or growth.8 On trichoscopy, the hair shaft should be evaluated for calibre and shape. The most notable feature of PHL is frontal predominant hair shaft thickness heterogeneity and increased number of miniaturised hair. Other hair shaft abnormalities that may be observed include exclamation mark hairs (AA, trichotillomania, and AE), comma or corkscrew hairs (tinea capitis), and coiled or flame hairs (trichotillomania).9,38
Specialised Tests
Hair pull test
The hair pull test is a non-invasive clinical exam that can be used to assess the presence of active hair loss. To perform the test, the clinician holds a hair bundle of approximately 30–40 hairs between the thumb and the index finger and gently pulls along the hair shaft from the scalp toward the hair ends. The test is considered positive if more than 10% of the hairs in each bundle are removed from the scalp area (i.e., unbroken hairs). A positive hair pull test at >1 scalp region (i.e., occipital, two parietal, and vertex) suggests TE or AE. 39,40
Tug test
The tug test, which is used to determine hair fragility, is a key component of hair shaft defect evaluations. To perform this non-invasive clinical exam, a hair bundle should be held in the middle of hair shaft while pulling on the end of the hair shaft. The tug test is considered positive if breakage of the hair shaft occurs, which indicates fragile hair and hair shaft abnormalities.1,8
Card test
To differentiate newly growing hairs from broken hairs, the card test may be used. To perform the card test, a card that contrasts in colour from the patient’s hair should be placed on the scalp against hair shafts in the affected area. Tapered ends indicate newly growing hairs and blunt ends indicate broken hairs.1,40
Scalp biopsy
Scalp biopsies are the gold standard for diagnosing cicatricial alopecia and can assist in the diagnosis of undifferentiated non-scarring alopecia.1,2 Ideally, an active area of the scalp with persistent hair fibres should be biopsied, and completely bald areas should be avoided.2
QUALITY OF LIFE TOOLS
To better characterise how hair loss affects the lives of patients, several standardised tests have been created for clinicians to administer to patients.41-44 Here, the authors highlight two studies that are most commonly used in clinical settings.
Hairdex Index
The Hairdex Index is a questionnaire that consists of 48 questions broken into five sections. Each of the 48 questions is self-graded on a scale of 0–4, with a score of 4 being the most severe change to a patient’s baseline quality of life.45 The five sections include symptoms, functioning, emotions, self-confidence, and stigmatisation. A 2021 study including 192 patients with AGA found that higher scores on the Hairdex were positively correlated with a diagnosis of anxiety and depression.46
Dermatology Life Quality Index
The Dermatology Life Quality Index (DLQI) consists of 10 questions, self-rated on a scale of 0–3.47 Though not specific to hair loss disorders, the DLQI has been validated in patients with AGA and AA. A study including 178 patients with AGA or AA found that patients were most like to report embarrassment, changes to social and leisure activities, and a lower ability to engage in working or studying.48
CONCLUSION
The psychosocial impact of hair loss on the quality of life of patients underscores the importance of dermatologist readiness to evaluate this common chief complaint. This review provides a systematic approach for dermatologists to use in order to accurately diagnose hair loss disorders.
When evaluating a patient presenting with new onset hair loss, the differential diagnosis may be broad, encompassing many subtypes of hair loss. Each type of scarring or non-scarring alopecia presents with its own unique aetiology, epidemiology, clinical presentation, trichoscopic findings, laboratory studies, and potential management tools. Furthermore, standardised quality of life tools for hair loss may increase awareness of the clinical significance of alopecia, which may foster earlier referral to dermatology and the development of innovative therapeutic treatment.






