Abstract
COVID-19 and cosmetic skin-fillers are two prevalent topics of today’s medicine, yet their interaction is not sufficiently studied. This article is based on a clinico-morphological case where the patient, a 37-year-old female, visited the clinic with complaints of painless palpable subcutaneous pathologic nodular lesions at the site of collagen cosmetic filler injection after severe acute respiratory syndrome coronavirus 2 infection. In order to verify the pathological processes of the lesions, punch biopsy of the affected skin was taken, and histological, histochemical, and immunohistochemical studies were conducted. Atrophy, acanthosis, parakeratosis with vacuolisation of nuclei of the epidermis; sclerosis and abnormal deposition of collagen fibres in the subepithelial layer of dermis; and vasculitis with endothelial hypertrophy and lymphoid perivascular infiltration (CD3 lymphocytes and CD68 macrophages) were found. Spike and nuclear capsid proteins of severe acute respiratory syndrome coronavirus 2 were localised in cells of perivascular inflammatory infiltrates, endothelial cells, and epithelium of glands and epidermis of the skin. The association between the dermatopathy in COVID-19 virus infection and cosmetic fillers were established. The authors discuss and hypothesise possible autoimmune processes that lead to autoimmune vasculitis.
Key Points
1. This article is based on a clinico-morphological case where the patient visited the clinic with complaints of pathologic formations at the site of collagen cosmetic filler injection after severe acute respiratory syndrome coronavirus 2 infection.
2. The patient was a 37-year-old female, who sought medical help for the complaints of painless palpable lesions in the cheek area, localised subcutaneously, persisting without dynamics. These blemishes were localised in the areas where, about 3 months prior, they had opted to inject collagen fillers for cosmetic purposes.
3. To verify the pathological processes of the lesions, punch biopsy of the affected skin was taken, and histological, histochemical, and immunohistochemical studies were conducted. The results and the implications of the findings are discussed in this article.
INTRODUCTION
The COVID-19 pandemic originated as a pneumonia outbreak in Wuhan, Hubei, China, in December 2019.1,2 By the 21st of October 2022, at least 623,893,894 people were affected, and 6,553,936 deaths were reported due to COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).3 Not only does COVID-19 affect the lungs, myocardium, kidneys, and central nervous system, but pathological changes have also been described in the skin.4-7 Singh et al.4 have described the prevalent occurrence of maculopapular lesions, urticarial lesions, and chilblain-like lesions (COVID toes) in COVID-19, while the other clinical manifestations of COVID-19 are vesicular lesions, petechiae, livedoid eruptions, and multisystem inflammatory syndrome in children with lesser degrees of occurrence.4,8,9
The use of dermal fillers, especially collagen fillers, for cosmetic purposes has been shown to be one of the most popular non-surgical cosmetic procedures in different populations of the world.10-13 The tissue changes after treatment with a dermal filler include increased collagen fibres expression with minimal angiogenesis, without changes in the epidermis and hypodermis.14,15 Increased incidences of acute inflammation relating to injectable collagen and hyaluronic acid fillers have been reported during the COVID-19 pandemic.16-20 Kato et al.16 have observed an association between COVID-19 and increased cases of acute inflammatory reaction in patients using collagen and hyaluronic acid dermal fillers for cosmetic purposes, and have hypothesised three probable causes of such correlation: rapid change in lifestyle, T helper 2 system activation due to stress, and immunologic reactions due to frequent exposure to cosmetic skin filler injection. Clinical manifestation of delayed inflammatory reaction to cosmetic dermal fillers have been observed and discussed.16,21 Still, pre-existing information on the interaction between cosmetic collagen skin fillers and COVID-19 is quite insufficient. Hence, studying the interaction between these two prevalent situations of today’s medicine, the COVID-19 pandemic and the use of cosmetic skin fillers, is necessary. This article is based on a clinico-morphological case where the patient visited the clinic with complaints of pathologic skin formations at the site of collagen cosmetic filler injection after SARS-CoV-2 infection. Written consent was obtained from the patient and this study was approved by the concerned ethical committee.
PATIENT INFORMATION
A 37-year-old female presented with complaints of painless nodular lesions in skin of their cheeks after about 8 weeks of first noticing them. The complaints were the appearance of painless palpable subcutaneous nodular lesions in the cheek area. These blemishes were located in the skin area where the patient had opted to inject collagen fillers for cosmetic purposes.22,23 These complaints were first observed 1 week after the patient tested positive for COVID-19. The statement provided by the clinic states that in the 12 calendar months preceding the presentation of lesions, the following drugs were injected into soft tissues of the face: a preparation based on hyaluronic acid (biorevitalizant) and based on polylactic acid, and preparations based on collagen and placental preparations. Procedures of botulinum toxin therapy and photodynamic therapy were also performed. According to the patient, the most recent and significant intervention was made a few weeks prior to testing positive for COVID-19. The intervention included mechanical cleansing followed by a meso-therapeutic administration of the injectable drug PDRN 6® (Cytolyfe, Austin, Texas, USA). The drug is composed of 2% polynucleotides (DNA) and 0.3% hyaluronic acid, which is unstabilised, with a molecular weight of 1.1. Dosages and administration regimens/schemes are unknown (Figure 1).
Figure 1: Anamnesis of the patient.
Upon clinical examination, status localis was noted as skin of normal physiological colouration, and lesions located in the lower third of the face and represented by tumour-like formations with clear boundaries and dense texture. Lesion foci of different diameters were localised in subdermal level. On the right side of the face in the region of the lower third, a single small papular inflammatory element was present (Figure 2A and 2B).
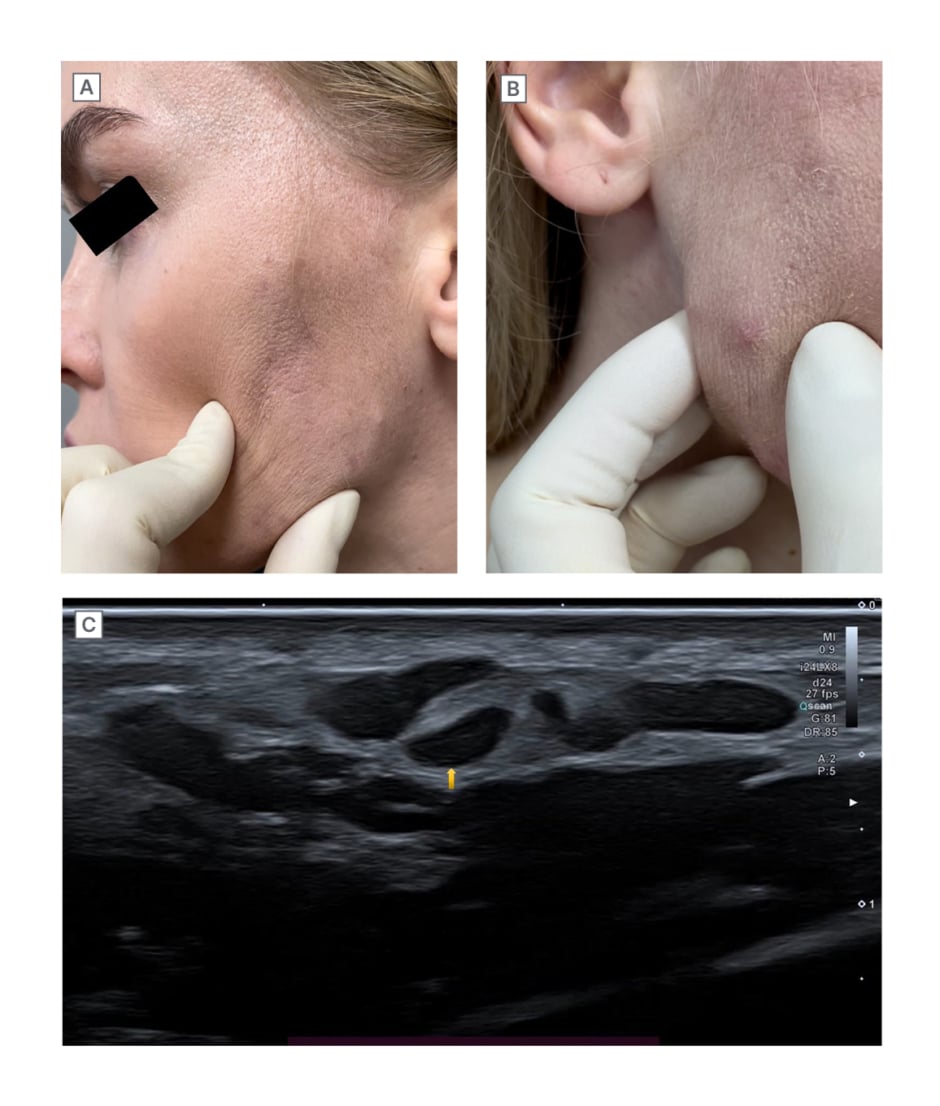
Figure 2: Clinical and radiological images on the day of presentation.
A) and B) Clinical pictures of nodular lesions on the day of presentation. C) Ultrasound image. Multiple hypoechoic nodular lesions, with clear contours in subcutaneous fat.
Ultrasound of soft tissues of the face revealed multiple palpable hypoechoic nodular lesions with clear even contours in the subcutaneous fat anterior to the zygomatic bones, caudally along the contour of the face. These formations were irregular in shape, diffusely heterogeneous, and were merging with each other. Intra- and peri-nodular vessels ranged from 1.40×0.90 mm to 16.00×3.15 mm in size. In some formations, single vessels were visualised. The echogenicity of the subcutaneous fat was diffusely increased, and blood flow was detected to be unevenly enhanced (Figure 2C). The main clinical diagnosis was post-injection granuloma of the skin and subcutaneous tissue caused by a foreign body. Localised oedema was the complication of the main diagnosis. It was histologically established that vasculitis was limited to the skin. Concomitant diagnosis was noted as acne, the papulo-pustular form, of mild severity.
A joint consultation with an allergist, an immunologist, and a general practitioner was held. It was recommended to examine and exclude vasculitis, since clinical signs of lymphocytic infiltration were observed. After additional laboratory diagnostics, there were no pathological changes found in general blood analysis or biochemical blood analysis; both tests were performed 9 weeks after being tested positive for COVID-19. Non-specific hypoallergenic diet was prescribed with the prescription of a tablet of Longidaza® 3000 (Petrovax, Moscow, Russian Federation) once per day and at night for 10 days, and one Trental® (Sanofi, Bridgewater, New Jersey, USA) 0.1 g tablet, three times daily after meals.
Following further examinations were recommended by the specialists: cyclic citrullinated peptide antibody test, antinuclear antibodies, antistreptolysin-O, and C-reactive protein to exclude an autoimmune component of rheumatoid arthritis and systemic vasculitis. Within 2 weeks of commencement of treatment, weakly pronounced positive changes were noticed in the form of a slight decrease in the size of the formations.
In order to verify the pathological processes of the lesions, punch biopsy of the affected skin was taken. The harvested skin sample was fixed in 10% formalin solution. Paraffin sections were prepared for histological, histochemical, and immunohistochemical studies.24
For histopathological study, the paraffin sections were stained with haematoxylin-eosin and Van Gieson’s staining.25 Other sets of paraffin sections were assigned for the characterisation of the histochemical markers: CD45 (Clone 4KB5, Dako, Agilent, Santa Clara, California, USA), CD3 (Clone MRQ-39, Cell Marque, Merck KGaA, Darmstadt, Germany), and CD68 (Clone Kp1 C, Dako).26,27 The expression of collagen-I (Clone 3G3, Santa Cruz Biotechnology, Dallas, Texas, USA), and collagen-III (Clone B-4, Santa Cruz Biotechnology) fibres were evaluated histochemically, and the tissue was examined for the presence of COVID-19 spike proteins (SARS-CoV-2 spike rabbit antibody, GeneTex Inc., Irvine, California, USA) and COVID-19 nuclear capsid (SARS-CoV-2 nucleocapsid rabbit antibody, GeneTex Inc.).28 Negative and positive controls were used.
Histological examination of the skin includes changes in epidermis and dermis. Atrophy, parakeratosis with vacuolisation of nuclei of the epidermis, and acanthosis were detected. Sclerosis and abnormal deposition of collagen fibres in the subepithelial layer of dermis were seen. Vasculitis with endothelial hypertrophy and lymphoid perivascular infiltration were found (Figure 3A).
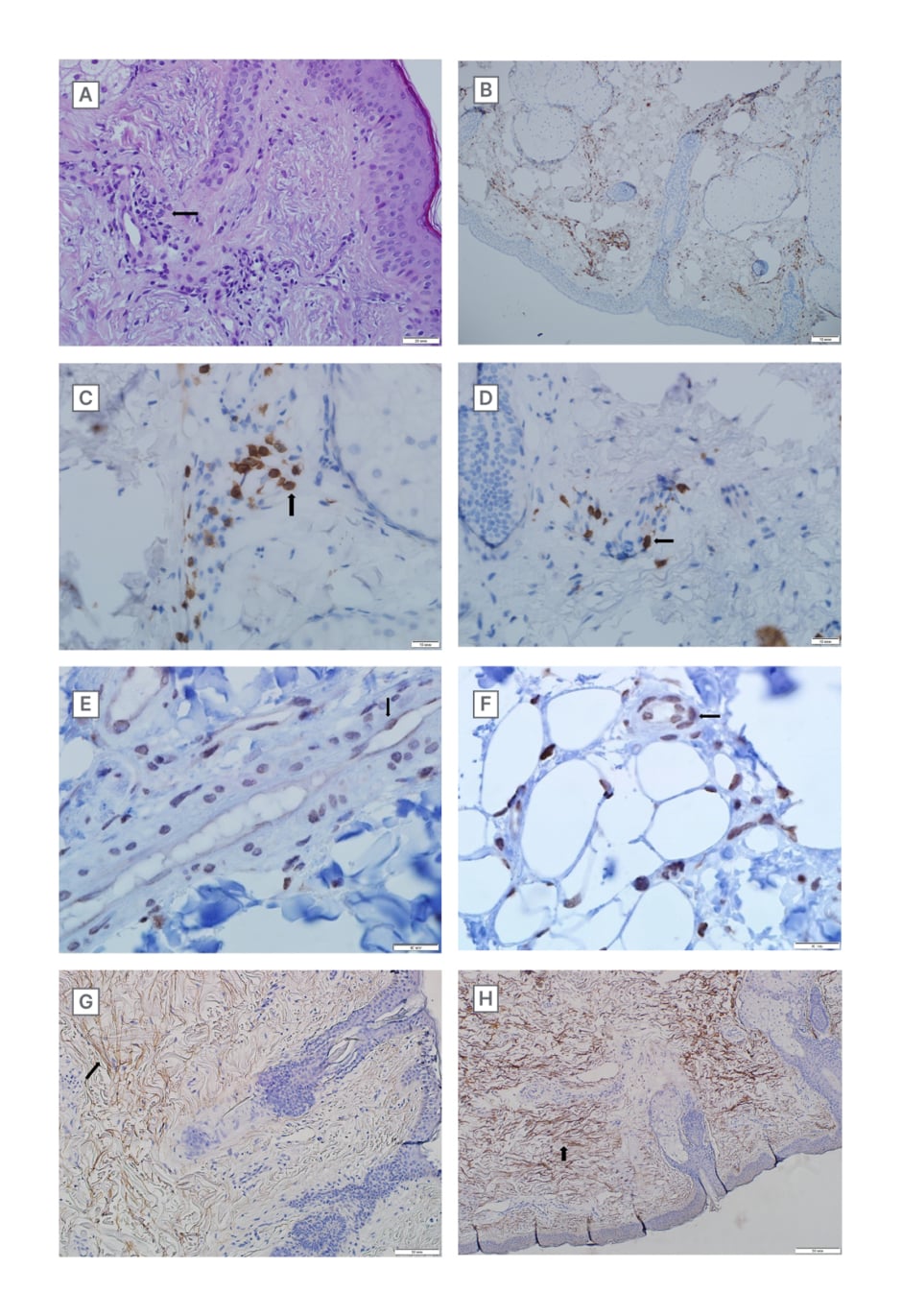
Figure 3: Microscopical images.
A) Sclerosis and vasculitis with endothelial hypertrophy and lymphoid perivascular infiltration in the derma, H&E (magnification 400X).
B) CD45 positive lymphocytes in dermal perivascular inflammatory infiltrate (Clone 4KB5, Dako, Agilent, Santa Clara, California, USA). Immunoperoxidase reaction with DAB. (magnification 600X).
C) CD3 positive lymphocytes in dermal perivascular inflammatory infiltrate indicating autoimmune reactions (Clone MRQ-39, Cell Marque, Merck KGaA, Darmstadt, Germany). Immunoperoxidase reaction with DAB (magnification 600X).
D) CD3 positive lymphocytes in dermal perivascular inflammatory infiltrate indicating autoimmune reactions (Clone MRQ-39, Cell Marque). Immunoperoxidase reaction with DAB (magnification 600X).
E) Nuclear capsids of the SARS-CoV-2 virus in endothelial cells and cells of perivascular inflammatory infiltrate (SARS-CoV-2 nucleocapsid rabbit antibody, GeneTex Inc., Irvine, California, USA). Immunoperoxidase reaction with DAB. (magnification 600X).
F) Spike proteins of the SARS-CoV-2 virus in endothelial cells and cells of perivascular inflammatory infiltrate. (SARS-CoV-2 spike rabbit antibody, GeneTex Inc.). Immunoperoxidase reaction with DAB (magnification 600X).
G) Collagen-I deposition in the subepithelial layer of derma (Clone 3G3, Santa Cruz Biotechnology, Dallas, Texas, USA). Immunoperoxidase reaction with DAB (magnification 200X).
H) Collagen-III deposition in the subepithelial layer of derma (Clone B-4, Santa Cruz Biotechnology). Immunoperoxidase reaction with DAB (magnification 200X).
DAB: 3,3′-diaminobenzidine; H&E: haematoxylin and eosin staining; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Immunohistochemical examination revealed dermal accumulation of collagen-I and collagen-III, with predominance of the former. Inflammatory infiltrates showed the presence of CD45 lymphocytes. CD3 lymphocytes were more than 10 cells in 1 mm of the slide, and CD68 macrophages were recorded as 20 cells in 1 mm of the slide.
Proteins of the COVID-19 virus, both spike protein and nuclear capsid protein, were localised in cells of perivascular inflammatory infiltrates, and in endothelial cells of vessels, as well as in the glands of the skin. In epidermis, cells with nuclear vacuolisation were detected to be positive for proteins of COVID-19 (Figure 3B–H). There were no data confirming a systemic autoimmune process in the patient. The anamnesis, according to the patient, the moment of high COVID-19 viral load coincided with the moment in time of mechanical cleaning of the face, and the administration of Cytolyfe PDRN 6.
DISCUSSION
The detection of COVID-19 spike proteins and COVID-19 nuclear capsid in the cells of perivascular inflammatory infiltrates in the endothelial cells and in the glands of the skin is an indication of some correlation between the COVID-19 and the skin lesions formed. This correlation can be due to either the direct effect of SARS-CoV-2 virus, or indirect effect.
The authors propose the direct effect, as the dermatopathy in this case was an immediate result of COVID-19 disease development in the patient. Earlier in the COVID-19 pandemic, the virus has been directly related with perivascular and peri-eccrine sweat gland lymphocytic. Predominantly CD3/CD4+ inflammation was observed in patients who had complaints with acral chilblain-like lesions and suspected SARS-CoV-2.29-36 There is substantial data about direct causation of Type III and Type IV hypersensitivities, vasculitis, and endotheliopathy after COVID-19 disease development.5,37-43 Formation of lesions can be due to some autoimmune reactions.44-50 Antiphospholipid autoantibodies were found in approximately half of the patients who were critically ill due to COVID-19 infection in two different studies.51-54 However, it must be noted that there were no data confirming a systemic autoimmune process in the patient.
Since the complaints were limited only the areas of prior administration with injections of the collagen skin filler, and the unnatural accumulation of collagen-III fibres was found in the intercellular matrix in the biopsy slide, the hypothesis that the injected collagen skin filler made the skin tissue more susceptible to such pathological processes can be made.55-57 The previously mentioned indirect effect can be due to such increased susceptibility of the skin tissue. Kato et al.16 have observed an association between COVID-19 and increased cases of acute inflammatory reaction in patients using the collagen and hyaluronic acid dermal fillers for cosmetic purposes, and have hypothesised three probable causes of such corelation: rapid change in lifestyle, T helper 2 system activation due to stress, and immunologic reactions due to frequent exposure to cosmetic skin filler injection. Clinical manifestation of delayed inflammatory reaction and hypersensitivity to cosmetic dermal fillers have been observed and discussed.16,21,55 Munavalli et al.58 also reported the significant increase of instances of acute inflammatory reaction after use of dermal filler in patients with a background of COVID-19. There have also been reports of delayed-type hypersensitivity reaction to non-hyaluronic acid polycaprolactone dermal filler following COVID-19 vaccination.17,59
CONCLUSION
The results of histological, histochemical, and immunohistochemical analysis of the punch biopsy, when viewed in parallel to the localisation of the lesion and patient’s anamnesis, have established that this dermatopathy is correlated both to COVID-19 infection and cosmetic collagen skin filler injection.
Though there are not enough scientific works to explain this dermatopathy completely, the two proposed explanations in this article, direct and indirect effects with development of autoimmune reactions, can each be supported to some extent by the pre-existing literature.






