Abstract
Chronic urticaria (CU) is characterised by the recurrence of hives/angioedema for >6 weeks. It affects children and adults and has a worldwide distribution. In children, CU is substantially less common than acute urticaria but is associated with larger decrease in quality of life. The current classification divides CU into two groups: 1) chronic spontaneous urticaria, which includes idiopathic urticaria (by far the most common type), autoimmune urticaria, and those associated with drugs, food, or additives allergies; and 2) chronic inducible urticaria, constituted by cholinergic urticaria and physical urticarias. Diagnosis of CU is based on the history and characteristics of the lesions. Although laboratory and specific testing could establish the diagnosis of some subtypes of CU, frequently the aetiology is never found; therefore, an extensive workup is not recommended. Once the trigger has been identified, it must be avoided. Specific treatment may be tried, but unfortunately this is not always possible. Currently, the first-line treatment for children with CU are second generation H1-antihistamines (SG-H1AH), such as cetirizine, fexofenadine, desloratadine, and rupatadine, among others. If, after 2–4 weeks, the patient has not improved, an increment from 2 to 4-times the regular dose is recommended. Patients that fail to respond to this treatment may be switched to another SG-H1AH or a second agent, such as H2-antihistamines (e.g., cimetidine, ranitidine), ketotifen, cyclosporine, or a leukotriene receptor inhibitor (e.g., montelukast), may be added to the H1-antihistamine therapy. Recently, omalizumab, an anti-immunoglobin-E monoclonal antibody has been approved in several jurisdictions for patients 12 years or older with recalcitrant CU; however, its high cost has limited its use.
INTRODUCTION
Urticaria is a common condition characterised by transient erythematous and oedematous plaques or papules with circumscribed erythematous borders and central clearing, known as hives/wheals. Hives are usually pruritic and their size and location varies, including mucosae. Urticaria can be accompanied by angioedema, which is characterised by poor delimited swelling (deeper oedema of the dermis and subcutaneous tissue) often involving the lips, tongue, eyelids, hands, and feet.1
Urticaria results from degranulation of dermal and submucosal mast cells/basophils that release vasoactive mediators such as histamine and lipid mediators (leukotrienes and prostaglandins), which enhance the expression of other cytokines and chemokines and induce an extravasation of fluid into the superficial tissues.2,3 Hives are the primary lesion of the urticaria but they can also be seen in other inflammatory conditions, such as urticaria pigmentosa, urticarial vasculitis, mastocytosis, and autoinflammatory syndromes; however, because these conditions are not considered within the classification of the urticaria due to their dissimilar pathophysiology, they will not be discussed in this review. Chronic urticaria (CU) is diagnosed when hives and or angioedema are present for >6 weeks.4
CLASSIFICATION OF CHRONIC URTICARIA
Based on the new classification of the Dermatology Section of the European Academy of Allergy and Clinical Immunology (EAACI), the EU-funded network of excellence, the Global Allergy and Asthma European Network (GA²LEN), the European Dermatology Forum (EDF), and the World Allergy Organization (WAO), CU is classified in two main groups:5
- Chronic spontaneous urticaria (CSU): hives and/or angioedema are present for >6 weeks with or without knowing aetiology.
- Chronic inducible urticaria (CIU): urticarial symptoms are triggered by specific stimuli.
EPIDEMIOLOGY AND AETIOLOGY
The frequency of urticaria (acute and chronic) in children is about 2.1–6.7%2 while CU prevalence ranges from 0.1–13.0%.6 It is estimated that 20–30% of children who initially present with autoimmune urticaria (AU) develop CU later on.7 Angioedema and hives are seen together in 50–80% of children with CU.8,9 A history of atopy or other allergic diseases can be present in up to 40% of this population.10,11 CU in children has a worldwide distribution, but does not seem to have a sex predilection.12
Although the precise pathogenesis of CU remains poorly understood, it is known that urticaria is the result of the mast cell/basophil degranulation triggered by a specific agent. Recently, the role of reactive oxygen species (ROS) and matrix metalloproteinase-9 (MMP-9) in the pathogenesis of CU has been investigated, as they are involved in the inflammatory processes. Dilek et al.13,14 found that children with CU had plasma levels of both ROS and MMP-9 higher than in healthy subjects and this had a positive correlation with disease activity.
Autoimmunity also plays a role in the pathogenesis of urticaria, since it has been found that at least 30–50% of patients with CU have circulating autoantibodies immunoglobulin (Ig)G against the alpha chain of the IgE receptor.6,15 Association of CU and autoimmune conditions, such as thyroiditis, is recognised in adults but in the paediatric literature seems contradictory; while some authors have found that 4–7% of children with AU had positive anti-thyroid antibodies;16 others have found no evidence of this association.12,17 Other autoimmune conditions that have been associated with AU include Type 1 diabetes mellitus, inflammatory bowel disease, systemic juvenile arthritis, systemic lupus erythematous, and coeliac disease.15,18
Infectious agents can also trigger CU. Several cases of Escherichia coli, Group A streptococcus, Chlamydia pneumoniae, cytomegalovirus, human herpes virus (HHV)-6, Epstein–Barr virus, and Helicobacter pylori have been reported.19,20 Recently, it has been proposed that HHV-6 and HHV-4 act as co-factors in the process of inflammation and autoimmunity observed in CU.21 The prevalence of parasitic infection (e.g. Blastocystis hominis, Giardia intestinalis, Dientamoeba fragilis, Enterobius vermicularis, Entamoeba spp.) in children with CU varies widely, from 1–10%, depending on the country of origin.10,22,23 Currently, the use of specific treatment for these infectious agents remains controversial, as in many cases CU symptoms persist or recur after the therapy has been discontinued.23,24
Allergies to drugs and food or food additives are well-known triggers of CU.25 Antibiotics and nonsteroidal anti-inflammatory drugs, including aspirin, are the main cause of drug allergies.3 Among CU patients with food and additives allergies, fruit, vegetables, seafood, colouring agents, preservatives (monosodium glutamate), and sweetener agents have been identified as the main culprits.19
Physical and cholinergic urticaria comprise a very particular subgroup of CU known as CIU. It is characterised by the development of hives or angioedema due to a specific physical stimulus such as heat, cold, pressure, vibration, water, ultraviolet light, etc.26 Rarely, patients may also have a mixed presentation with more than one type of CU.27
The literature regarding CU prevalence in children is scarce compared to that of adults; however, a recent systematic review revealed that CSU was the most common type of CU (85%) while patients with CIU represented only 15% of the cases. Within the group of CSU, >55% of the patients had an unknown aetiology (idiopathic), 28.4% had AU; allergies to drugs and foods/additives were found in 25%, and infections were identified in 4.5% of cases.23 Azkur et al.16 performed a prospective study in 222 children with CU of which 59.9% had CSU and 40.1% CIU. Within the CSU group, 53.5% had AU, 32.8% had positive 14C-urea breath test for H. pylori, and 6.5% had positive stool test for parasites. In the group of CIU, 77.5% had dermographism, 16.8% had cold urticaria, 2.2% had both cholinergic urticaria and solar urticaria, and aquagenic urticaria was present in 1.1% of the patients.16 In another study focussed solely on paediatric CIU (N=53), 38% had dermographism, 19% had cholinergic urticaria, 17% had mixed physical subtypes, 9% each had pressure inducible urticaria, cold urticaria, and heat urticaria, and 4% were unspecified.28
CLINICAL PRESENTATION
Chronic Spontaneous Urticaria
In general, patients with CSU present with frequent hives for >6 weeks, which usually resolve within 24 hours, leaving no mark. Oropharyngeal oedema or angioedema can be seen in ≤80% of patients, but seldom represents a life-threatening condition.7 In patients with IgE-mediated food or drug allergy, the symptoms are not evident after the first hour after the exposure.⁹ Although presentation of parasitic infection-related CSU (PIRCSU) is comparable to non-PIRCSU, the former seems to affect younger children (3–12 years) and the length of the disease is shorter. Also, the prevalence of gastrointestinal symptoms (nausea/abdominal pain) are substantially higher.22 Overall, patients with AU have no clinical difference among children with non-AU. A couple of studies in children have not been able to find a clinical correlation among levels of antibodies and severity or chronicity of urticaria.11
Chronic Inducible Urticaria
Cold Urticaria
Cold urticaria presents with redness, swelling, and itching in unprotected areas of the skin exposed to cold water, ice, or being outdoors in cold weather. The symptoms appear after a few minutes and once the stimulus has been avoided, they resolve within 30–60 minutes. Systemic symptoms, such as headache, fatigue, feeling light-headed, vomiting, or anaphylaxis, are present in ≤50% of patients after generalised cold exposure (e.g. swimming). Association with a recent cryoagglutinins-associated viral infection has also been reported.29
Heat Urticaria
In contrast to cold urticaria, in heat urticaria hives develop 10 minutes after contact with a heat source (45°C) for ≥5 minutes.26
Dermographism
Dermographism is characterised by erythema and/or swelling occurring at the sites of friction or minor trauma (e.g. scratching, clothing, clapping).
Usually, hives are localised on the surface of the skin and clear after 30–60 minutes. Association with angioedema is rare.2,26
Solar Urticaria
Patients develop abrupt onset of erythema, hives, itching, and sometimes angioedema in areas that have been exposed to sunlight (ultraviolet light-A and, less frequently, ultraviolet light-B) or other visible light sources. The reaction occurs during or after a few minutes of the exposure, but there can be a latency period of several hours between the irradiation and the first appearance of any symptom.30 Once the exposure ceases the symptoms resolve after 30 minutes or within the first 24 hours. Interestingly, the wavelength of light that triggers the symptoms varies in each patient. Rarely, full body exposure might lead to systemic symptoms and anaphylaxis.31,32
Vibratory Urticaria
Rarely seen in children, this type of urticaria is characterised by pruriginous erythema or swelling at the site of a vibratory stimulus, such as running, motorcycling, or electric tools (e.g. lawnmower, pneumatic drill). The symptoms usually appear within minutes of vibration and last several hours after the stimulus has been discontinued. When the stimulus continues, systemic symptoms, such as facial flushing, chest tightness, and an associated generalised feeling of heat, may develop.33 This urticaria can be caused by a mutation in the ADGRE2 gene, which presents with an autosomal dominant inheritance.34
Delayed Pressure Urticaria
Unlike the other physical urticarias, delayed pressure urticaria consists of the development of hives/swelling from 30 minutes up to 9 hours after exposure to pressure, such as tight clothing, hammering, sitting down, or carrying heavy shopping bags. Skin lesions also last longer (12–72 hours) than in other types of physical urticaria.35 Any part of the body may be affected, but the palms, soles, lips, shoulders, arms, and buttocks are usually more frequently involved. Pruritus may be absent or mild; instead patients describe localised pain and a burning sensation.36 Extracutaneous manifestations, such as flu-like symptoms and arthralgia, may accompany skin lesions.37
Cholinergic Urticaria
Although it is included within the subgroup of CIU, it is not considered a physical urticaria because it is triggered by a rise in temperature of the body core/sweating, rather than an exogenous physical trigger acting on the skin to induce the symptoms.5 Cholinergic urticaria presents as very pruritic point-size papules or hives rapidly after exercising, emotional distress, hot bath, or spicy food, and usually clears within 1 hour.38 The skin lesions may involve any part of the body; however, the neck, flexor surfaces of the elbows, knees, wrists, and inner thighs are more frequently affected.7 Like exercise-induced urticaria/anaphylaxis, cholinergic urticaria can be triggered by physical activity, but they might be different entities.2 Hives in exercise-induced urticaria are usually larger and the risk of anaphylaxis is higher. Angioedema and systemic symptoms can also be present in patients with cholinergic urticaria, but is uncommon.39
Aquagenic Urticaria
Aquagenic urticaria manifests as extremely small pruriginous papules/hives, mostly localised to the neck, upper trunk, and arms. They develop within 5–20 minutes of contact with fresh or salted water, regardless of its temperature.40 It is rarely seen in small children and it usually develops after puberty (mean age: 11–49 years). Although it presents in both sexes, it seems to be more common in females.41 A familial presentation has also been reported.42 Systemic symptoms might present if a large body surface area is submerged for several minutes. Hives last around 20–30 minutes and resolve spontaneously.43
ASSESSMENT OF SEVERITY AND QUALITY OF LIFE IN PATIENTS WITH CHRONIC URTICARIA
Although CU could occasionally represent a life-threatening condition, most of the time symptoms, such as pruritus, hives, or angioedema, resolve promptly without severe complications; however, the recurrence of such symptoms can represent a heavy burden for children and their families. The recent EAACI/GA²LEN/EDF/WAO guidelines for urticaria recommends using the urticaria activity score for 7 days to evaluate the severity of hives and itch in patients with CU. Every day, patients must assign a value from 0–3 to the intensity of the itch (0: none, 1: mild, 2: moderate, 3: severe) and to the number of hives (0: none, 1: <20 hives, 2: 20–50 hives, 3: >50 or large hives). The final score ranges from 0–42. A higher score represents greater severity.5 Visual analogue scales have also been used to evaluate disease severity in adults and children with CU.44
As can be expected, the quality of life (QoL) of children with CU is impaired from a greater to a lesser degree depending on disease severity. Daily activities such as school performance, sleep, personal care, and peer interaction can be affected not only by the symptoms but also by side effects of treatment.19 Despite the fact that few QoL studies in children with CU have been performed, it is known that CU in children affects QoL similarly to other chronic cutaneous conditions, such as atopic dermatitis.45 Currently, the Chronic Urticaria Quality of life Questionnaire is the only specific tool that evaluates the severity and impact on QoL in patients with CU.46 Other instruments used in children with CU include Children’s Dermatology Life Quality Index, Dermatology Specific Quality of Life, Skindez-29, and the Urticaria Severity Score (not validated in children).44,47
DIAGNOSIS
Diagnosis is established based on the history and clinical characteristics of the cutaneous and systemic manifestations. Extensive laboratory testing is usually unnecessary and should be guided by symptoms or suspicion of an underlying condition (Figure 1).48 Either autologous serum skin test (ASST) or basophil activation test (BAT) can be used to diagnose AU.17 Both tests have a high sensitivity and specificity to detect basophil histamine releasing activity. While ASST is an in vivo test, BAT is performed in vitro. Recently, Netchiporouk et al.49 showed that high levels of BAT in children with CU are statistically significantly associated with a higher disease activity. CIU can be diagnosed by specific challenge testing.50 Skin biopsy is rarely necessary, but it can rule out other conditions when systemic symptoms and skin lesion are not consistent with any type of CU.9
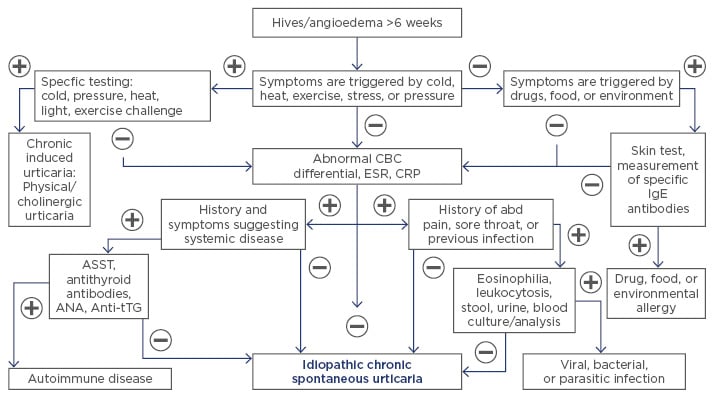
Figure 1: Diagnosis of chronic urticaria.
abd: abdominal; ANA: antinuclear antibody; ASST: autologous serum skin test; Anti-tTG: anti-tissue transglutaminase antibody; CBC: complete blood count; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IgE: immunoglobulin E.
TREATMENT
The goal of treatment is to eliminate the symptoms or reduce their severity/frequency. Eradication or avoidance of triggers (if identified) is the first step;7 however, in many cases this is not possible or symptoms continue despite specific treatment (antibiotics, anti-parasitic drugs, thyroid hormone replacement).
The first-line treatment for CU is antihistamine therapy, as it inhibits the effect of mast cell and basophil mediators on the target tissues.48 First-generation H1-antihistamines may temporarily relieve the symptoms of CU; however, they are no longer recommended due to undesired side effects (sedation, impairment of alertness and cognition).2 However, some authors have reported the efficacy and safety of ketotifen (a non-competitive H1-antihistamine and mast-cell stabiliser) in patients with CU.51
The new EAACI/GA²LEN/EDF/WAO guidelines strongly support the use of second-generation H1-antihistamines (SG-H1AH) for CU.5 In children, several studies regarding the safety and efficacy of cetirizine, levocetirizine, loratadine, fexofenadine, desloratadine, and rupatadine have been performed.52 The regular dosage of SG-H1AH could be increased up to 2–4 times if symptoms are not resolved or improved within the first 2–4 weeks of treatment; if there is still no improvement, another H1-antihistamine could be tried.53 A few studies performed in children with CU have shown that around 35–38% of these patients required double doses of SG-H1AH, while only 6% and 5% needed triple or quadruple dosage, respectively. Interestingly, younger children seem to respond better to regular doses, while older children require higher doses to achieve resolution of their symptoms.53,54 Adding an H2-antihistamine (cimetidine, ranitidine) for patients who still have not been able to achieve complete remission of their symptoms has been recommended by some authors, although this remains controversial.3 For those who have failed monotherapy, next line of treatment includes adding a leukotriene antagonist (LTRA), short course of oral corticosteroids, cyclosporine, or omalizumab.6
LTRA, such as montelukast and zafirlukast, have been used successfully in children with asthma or other allergic diseases; however, literature regarding paediatric CU is almost non-existent. In adults, a recent systematic review showed controversial results. As monotherapy, LTRA were better than placebo, but less effective than SG-H1AH, while combined therapy (SG-H1AH+LTRA) showed overall better results.55-57 Currently, the use of corticosteroids in CU is restricted only for exacerbations and for short periods of time (3–7 days), due to their well-known side effects.2,5,52
Cyclosporine has also been used as adjuvant therapy in the treatment of difficult to control CU.58 Moreover, it has been shown that cyclosporine is more effective in patients with elevated high-sensitivity C-reactive protein. In children, few studies have showed its effectiveness.59 Doshi et al.60 reported seven patients treated with cyclosporine after failing high-dose SG-H1AH and corticosteroid therapy. All patients reached cessation of the symptoms within the first 8 weeks of treatment, with no evidence of side effects.60 Although uncommon, side effects (e.g. infections, hypertension, nephrotoxicity, headache, nausea, abdominal pain) have been reported. Thus, a close monitoring of blood pressure, renal function, and cyclosporine levels is recommended.61
Omalizumab is a recombinant DNA-derived humanised monoclonal antibody that binds to the constant region of the IgE molecule, avoiding the interaction between free IgE with high and low-affinity IgE receptors. This reduces the levels of IgE and leads to a downregulation of high-affinity IgE receptor expression on inflammatory cells.62 Lately, the interest in omalizumab as second-line therapy for patients with CU has increased significantly, because several studies and case reports have showed its effectiveness and safety in both adults and children.63-65 In children, omalizumab is currently approved for moderate-to-severe uncontrolled allergic asthma and CSU (≥12 years). Off-label uses include allergic rhinitis, food allergy, and anaphylaxis, and severe refractory and atopic dermatitis.66 Improvement of the symptoms can be observed from the first dose; however, to prevent recurrence and obtain complete remission, at least 5 doses are considered necessary.65 Overall, omalizumab seems to be safe with few side effects reported (mainly mild skin reactions at the injection site and, in rare cases, anaphylaxis), but its high cost has limited its use.12,66-68 Please see Figure 2 for further information.
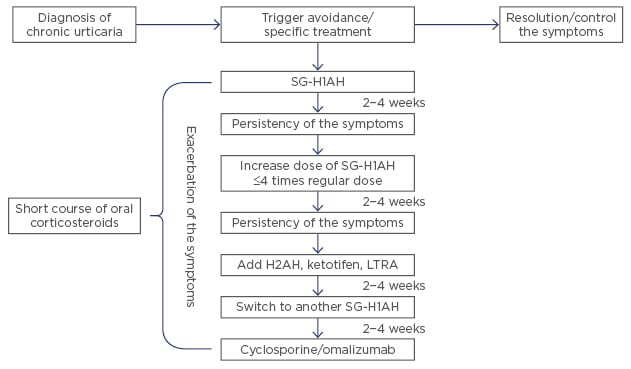
Figure 2. Treatment steps for children with chronic urticaria.
Note: this flowchart is a summary of recommendations by several authors included in this review. It is not intended to replace the current guidelines by the EAACI/GA² LEN/EDF/WAO.
H2AH: H2-antihistamine; LTRA: leukotriene antagonist; SG-H1AH: second generation H1-antihistamines.
PROGNOSIS
Most children with CU achieve improvement or control of their symptoms within the first 5 years after the diagnosis, although some of them continue to experience the symptoms even longer. However, studies performed in children have dissimilar results. While some authors report a remission rate at one year of 84%, others report only 16–18% for the same period.69,70 Overall, by the end of Year 5, between 38.4% and 67.7% of the patients are free of symptoms.10 Children with CIU and AU seem to have symptoms for longer, but this is also a controversial suggestion as some authors have found no difference among these patients.2,19
CONCLUSION
CU in children is uncommon but represents a challenge for many physicians. The recurrent episodes of hives or angioedema impact the QoL of children and their parents. The diagnosis is based on the clinical characteristics of the symptoms. Extensive work-up is rarely necessary, but specific testing guided by a good history can confirm the diagnosis in many cases. The first line of treatment continues to be SG-H1AH. The emergence of new therapies, such as omalizumab, could represent a hope for patients with recalcitrant CU.
Appendix
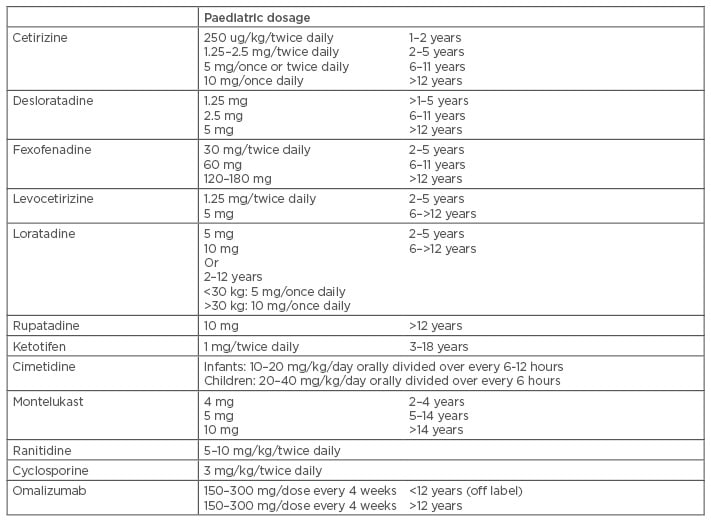
Table 1: Medications for chronic urticaria in children.1,52,60,68,71







