Abstract
Background: Botulinum toxin (BoNT), a bacterially produced neurotoxin, is a mainstay in the dermatologic armamentarium. Although BoNT is commonly used to treated rhytides associated with ageing, it can be employed for a variety of other cosmetic purposes and medical disorders.
Objective: In this review, the authors aim to describe the multitude of uses for BoNT in the dermatologic field.
Materials and Methods: This manuscript was designed as a retrospective review of the on- and off-label applications of BoNT in dermatology.
Results: In addition to treatment of rhytides, BoNT has been shown to decrease rosacea, menopause-associated flushing, and facial sebum production, while improving patient confidence in their appearance. Furthermore, BoNT has been successfully used to treat primary hyperhidrosis, hair loss, aberrant scarring, Raynaud’s phenomenon-associated vasospasm, as well as a variety of skin diseases. Side effects of BoNT include pain or discomfort associated with injections during treatment, bruising, asymmetry, and swelling. Patients are generally satisfied with clinical results after BoNT treatment.
Conclusion: Dermatologists should be aware of all on- and off-label applications of BoNT to provide patients with timely and appropriate medical care. Further research must be completed to fully characterise the safety and use of BoNT for off-label purposes.
INTRODUCTION
Botulinum neurotoxins (BoNTs) are derived from Clostridium botulinum, a gram-positive anaerobe. While there are seven subtypes of BoNT (A to G), only A and B are currently clinically relevant. The cosmetic potential of BoNT-A was first described in the early 1990s by Drs Jean and Alistair Carruthers as a safe and effective treatment for dynamic rhytides.1 Ten years later, BoNT-A was approved by the U.S. Food and Drug Administration (FDA) for the treatment of glabellar rhytides. Since their commercial availability, BoNTs have been used on- and off-label in both the medical and cosmetic realms for spasticity, migraines, depression, hyperhidrosis, as well as ageing of the face, neck, and décolletage. BoNT is secreted by C. botulinum as a three-protein complex with the 150 kDa toxin, a non-toxin haemagglutinin protein, and a non-toxin, non-haemagglutinin protein. Bacterial proteases cleave the toxin into an active, di-chain product consisting of the 100 kDa ‘heavy’ and 50 kDa ‘light’ chains. Once the active toxin is introduced to the presynaptic nerve terminal, the heavy chain binds synaptic vesicle glycoprotein 2 causing endocytosis of the toxin–glycoprotein complex and toxin light chain release into the synaptic space. Toxin light chains cleave either synaptosomal-associated protein 25 (BoNT-A, C, E), or vesicle-associated membrane protein/synaptobrevin (BoNT-B, D, F, G), disallowing the release of acetylcholine from the axon of peripheral motor nerves, and causing subsequent temporary chemical denervation and muscle paralysis.2
There are four commercially available, FDA-approved BoNT-A preparations in the USA: onabotulinumtoxinA (ONA; Botox®, Allergan Inc., Irvine, California, USA); abobotulinumtoxinA (ABO; Dysport®, Medicis Pharmaceuticals Corp., Scottsdale, Arizona, USA); incobotulinumtoxinA (INCO; Xeomin®, Merz Aesthetics, Frankfurt, Germany); and, newest to the market, prabotulinumtoxinA-xvfs (PRA; Jeuveau™, Evolus, Newport Beach, California, USA); as well as one BoNT-B: rimabotulinumtoxinB (RIMA; Myobloc®, Solstic Pharmaceuticals, San Francisco, California, USA). While ONA, ABO, and INCO are approved for use in the medical and cosmetic settings, PRA is only approved for glabellar lines and RIMA is only approved for the treatment of cervical dystonia (Table 1). For the purposes of this present review, it is important to note that all treatments discussed will refer to units (U) of BoNT-A as ONA equivalents, unless otherwise stated.
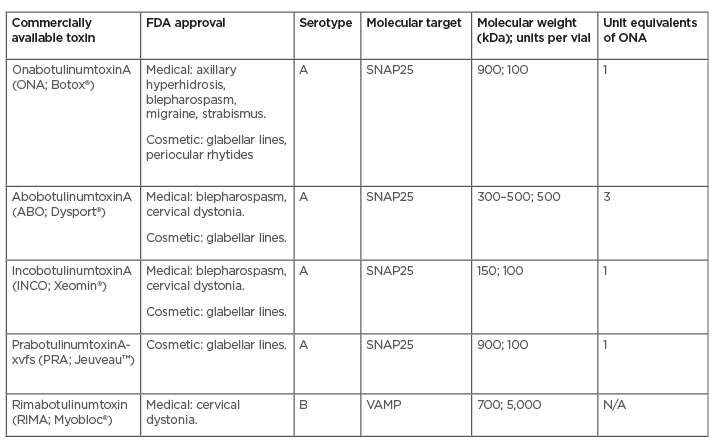
Table 1: Characteristics of commercially available botulinum toxins in the USA.
N/A: not applicable; ONA: onabotulinumtoxinA; SNAP25: synaptosomal-associated protein 25; VAMP: vesicle- associated membrane protein.
SAFETY
The human medial lethal dose of BoNT-A is estimated at 1.3–2.1 mg/kg (intramuscularly or intravenously), correlating to a median lethal dose (LD50) of 26–42 units/kg;3 thus, at the doses used for cosmetic and medical treatment, BoNT is considered safe. Though contraindications to BoNT injection are few, they exist, and include allergy to constituents of BoNT because of the potential for anaphylaxis; neuromuscular disorders such as myasthenia gravis, Lambert-Eaton syndrome, and amyotrophic lateral sclerosis; and pregnancy and nursing. Although medications such as cyclosporine, D-penicillamine, and aminoglycosides are not contraindicated, a thorough medication history is warranted as these drugs may cause neuromuscular junction abnormalities and possibly potentiate the effects of BoNT.4
The most common side effects of BoNT administration are pain or sensitivity in the area injected, bruising, swelling, and asymmetry. During injection, it is important that injectors are aware of surrounding musculature as diffusion of BoNT may affect areas that were not intended for treatment. In the literature, the majority of reported adverse events occur after cosmetic treatment of the head and neck for rhytides. Headaches can occur in up to 11.4% of BoNT-A-treated patients, considered to be more likely a result of trauma secondary to injection rather than the BoNT itself, as 20% of placebo patients also report headache. Headaches can arise up to 2 days post-injection and persist for 2–4 weeks.5 When injecting into the platysma, clinicians should assess patients for dysphagia as this is a rare but serious complication, especially when using high doses of BoNT-A.6 Generalised reactions to BoNT have been reported in clinical trials, including flu-like symptoms, malaise, nausea, and distant cutaneous reactions such as dermatitis; however, these adverse events are uncommon and self-resolve.7
Although immunogenicity to the 150 kDa toxin of BoNT-A was originally reported, it is rare in patients treated after 1997. New formulations of BoNT-A contain only 20% of the original protein and are hypothesised to decrease the likelihood for patient immune reaction. However, cases of immunogenicity even to newer BoNT-A formulations are occasionally reported; for instance, a patient developed neutralising antibodies after treatment of masseter hypertrophy using Vistabel® (Allergan Inc., Irvine, California, USA) and Azzalure® (Galderma S.A., Paris, France). Risks for increased immunogenicity include large BoNT-A doses, frequent injection, and short intertreatment duration (<3 months).7
MATERIALS AND METHODS
A systematic review of the PubMed database using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was completed in December 2020 using the search term “botulinum toxin AND (flushing OR erythema OR rosacea OR menopause OR oily OR sebum OR rhytides OR wrinkles OR lines OR mood OR hyperhidrosis OR alopecia OR bullous OR Hailey-Hailey OR pemphigus OR linear IgA OR epidermolysis bullosa OR scar OR keloid OR Raynaud OR hidradenitis suppurativa OR psoriasis),” which resulted in a total of 2,951 total articles. Inclusion criteria were manuscripts written in English pertaining to the use of botulinum toxin in dermatologic conditions in the past 10 years; review papers, commentaries, editorials, practice guidelines, animal studies, and articles not written in English were excluded from review.
RESULTS
After inclusion and exclusion criteria were applied, 404 studies were reviewed, with 208 discussing cosmetic uses of botulinum toxin such as rhytides or facial rejuvenation (n=185), flushing (n=13), depression or psychologic effects after rhytide treatment (n=7), and oily skin (n=3); while 196 discussed medical uses including hyperhidrosis (n=100), abnormal scarring (n=32), Raynaud’s phenomenon (n=25), bullous disease (n=10), alopecia (n=5), as well as other disease processes (n=24).
Cosmetic Uses
Flushing
Flushing or erythema of the scalp, face, neck, and chest can be caused by a variety of underlying neurogenic conditions such as rosacea or symptomatic menopause. Although the use of BoNT has mixed results for the treatment of flushing, multiple studies demonstrate that the injection of 10–500 U of BoNT-A over affected areas in a 1 cm grid-like pattern results in decreased symptomatology (such as ‘hot flashes’ or perspiration) and improved patient quality of life as measured by the Dermatology Quality of Life Index (DLQI) over 6 months.8,9
Oily skin
BoNT-A has been trialled for the treatment of excess sebum production of the forehead and ‘T-zone’. Although it is not known how BoNT affects sebaceous glands, it is possible that the toxin targets local muscarinic receptors and arrector pili muscles, thus regulating sebum production. Preliminary retrospective data show that patients treated with BoNT report decreased sebum production and pore size. A more recent prospective study injected 30–45 U of ABO to the foreheads of 25 subjects, with 91% of patients reporting a decrease in sebum production and a reduction in pore size after assessment of photographs.10
Rhytides
Overactive musculature, photodamage, and ageing cause the formation of dynamic and static rhytides (i.e., ‘wrinkles’) that patients perceive as making their appearance fatigued or angry; treatment of facial rhytides can lead to a more relaxed and rested appearance. Although currently the FDA has only approved BoNT for treatment of glabellar (corrugator supercilii, depressor supercilii, and procerus muscles) and periorbital lines (‘crow’s feet’; orbicularis oculi muscle), off-label use of BoNT occurs for the treatment of horizontal forehead lines (frontalis muscle), ptotic brow (lateral orbicularis oculi), horizonal nasal lines (‘bunny lines’; nasalis muscle), ‘gummy smile’ (levator labii superioris alaeque nasi muscle), perioral lines (orbicularis oris muscle), dimpled chin (mentalis muscle), ‘downturned smile’ (depressor anguli oris), masseter hypertrophy (masseter muscle), mandibular border (‘Nefertiti lift’; superior platysmal band), and platysmal bands of the neck (platysma muscle), with clinical results lasting approximately 3 months (Figure 1).6,11
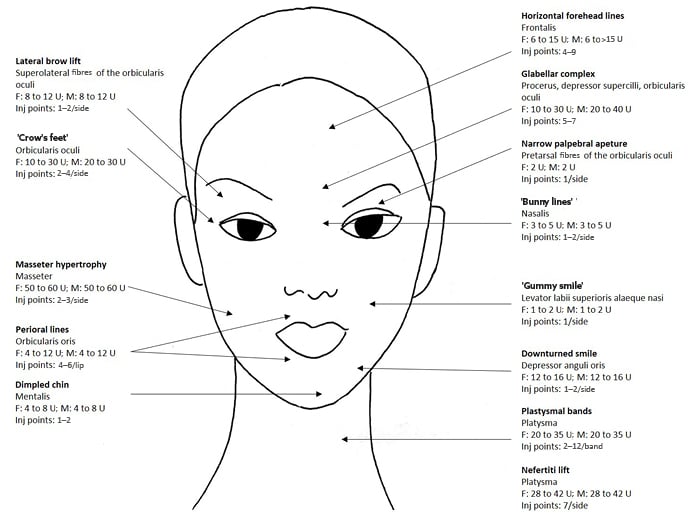
Figure 1: Target areas for BoNT-A treatment of the face.
BoNT-A treatment of rhytides of the upper, mid, and lower face, with suggested total ONA units for female and male patients, as well as number of injection points.32
BoNT: botulinum toxin; F: female; inj: injection; M: male; ONA: onabotulinumtoxinA.
Combination therapy
Combining BoNT with filler injection for simultaneous treatment of dynamic rhytides and volume loss is popular in the outpatient setting. Hyaluronic acid and calcium hydroxyapatite fillers in combination with BoNT have been used successfully in multiple areas of the face, including the glabella, periorbital or perioral areas, nasolabial folds, as well as the jaw and chin. Filler should be placed before BoNT to avoid migration of toxin that may occur when injected filler is massaged. It has even been suggested that the combination of filler and BoNT can increase the duration of filler clinical efficacy in the glabellar area to almost double because of decreased facial movement.5
BoNT should be avoided on the same day as laser treatment because of concerns for toxin diffusion. Although no studies have shown BoNT diffusion after microfocused/high-intensity focused ultrasound or radiofrequency microneedling treatment, it is reasonable to assume that diffusion may occur after such modalities and the same caution should be considered.
Psychological improvement
In conjunction with rhytide reduction, BoNT improves patient mood and perceived confidence. Improvements in the FACE-Q (satisfaction with facial appearance, satisfaction with facial skin, and appraisal of facial lines) score after treatment of moderate-to-severe glabellar lines have been reported. Even after 120 days, when clinical effects of BoNT should be waning, patients have reported increased psychological well-being and improved facial appearance.
Given the impact of BoNT impact on mood lasting beyond its effect on musculature, clinicians should discuss with patients at which point retreatment is necessary to achieve maximal psychological and clinical response, rather than automatically reinjecting BoNT every 3 months.12,13 In addition, BoNTs have been used in neurology for the successful treatment and prevention of migraines, with a notable increase in patient well-being and quality of life.14
Medical Uses
Within the purview of dermatologic practice, the only FDA-approved medical use of BoNT is ONA for the treatment of axillary hyperhidrosis. Despite further lack of approval, BoNT has been used off-label for the treatment of alopecia, bullous skin disorders, palmar and plantar hyperhidrosis, hypertrophic and keloidal scarring, as well as other dermatologic medical conditions such as Raynaud’s phenomenon, hidradenitis suppurativa, and psoriasis.
Hyperhidrosis
BoNT disrupts acetylcholine-induced secretion from eccrine sweat glands, which are located in the axillae, as well as throughout the body. ONA is approved by the FDA for the treatment of primary axillary hyperhidrosis. The use of BoNT for treatment of primary hyperhidrosis has extended to use in the palms and soles, as well as other areas of the body. Adolescent patients as young as 12 years old have been treated successfully with BoNT, with some researchers advocating for early treatment of hyperhidrosis to decrease future patient morbidity such as decreased quality of life.15
Treatment of the primary axillary hyperhidrosis can be accomplished using 50–100 U of BoNT-A per axilla with intradermal injections spaced every 1–2 cm in a grid-like pattern (for approximately 15 injection sites). Clinical results are noticeable after 1 week and last for 3–10 months. Patients are generally satisfied with treatment. It is important to inform patients that compensatory sweating can occur in up to 5% of cases post-injection.4,16,17 Treatment of palmar and plantar hyperhidrosis can also be accomplished with BoNT. Injections should be spaced 1 cm apart in a grid-like pattern with 2–3 injection points per digit (for approximately 35–50 injection sites). Each hand can receive 75–100 U of BoNT-A, while each foot can receive 100–200 U. Clinical results take up to 1 week for effect and last 3–6 months. Specific adverse events associated with palmar and plantar injection of BoNT should be discussed with patients prior to treatment. After palmar injection, patients can experience weakness, especially in pinch strength, while plantar injections may impede walking, especially if a nerve block is performed prior to BoNT treatment.18,19 Unfortunately, 20% of patients injected with BoNT for plantar hyperhidrosis will not respond to treatment (Table 2).
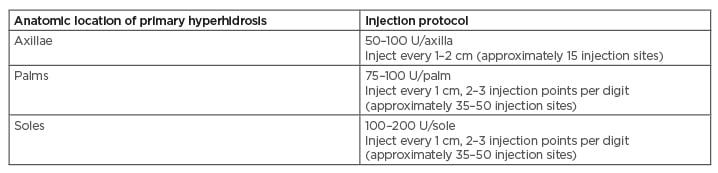
Table 2: Protocols for the treatment of primary hyperhidrosis using BoNT with suggested total ONA units, as well as number of injection points.19
BoNT: botulinum toxin; ONA: onabotulinumtoxinA.
Due to the painful nature of injections into the palms and soles, discomfort during BoNT injections has been mitigated in a variety of ways. Studies have demonstrated that topical lidocaine/prilocaine, vibration, cryoanalgesia, repeated needle replacement, use of microneedles, intravenous administration of anaesthesia and peripheral nerve blocks, iontophoretic administration of 2% lidocaine, needle-free units to administer local anaesthesia using pressure, and inhaled nitrous oxide are all viable methods of analgesia during injection.17,19-25 Advances in BoNT delivery may also decrease injection-associated pain. Needle-free jet injection devices have been trialled for the treatment of palmar hyperhidrosis (Med-Jet BMX, Medical International Technologies, Montréal, Canada); however, clinical efficacy was decreased as compared to traditional injections.26 An in vivo murine study demonstrated that BoNT-A-coated polylactic acid microneedles are a safe and effective delivery method, resulting in significantly decreased sweating.27 Delivery of BoNT-A using a combined fractional ablative laser and ultrasound has been trialled successfully to treat palmar hyperhidrosis.28 A topical formulation of BoNT-A in a liposomal base applied nightly for 7 nights was found to be effective at reducing axillary sweat for up to 8 weeks, compared to vehicle alone, suggesting that painless delivery methods for BoNT are on the horizon.29
Recent studies have employed BoNT to treat hyperhidrosis in novel locations. For example, one case report injected 100 U of BoNT-A every 6–8 months at the gluteal cleft of a male patient with pressure-induced ulceration to decrease sweat production and associated wound maceration; over a period of 2 years, skin integrity was maintained with no clinical worsening of the pressure injury.30 Another study employed a total of 2250 U of BoNT-B (Neurobloc, Eisai Europe, Hatfield, UK) injected every 15 mm across the forehead, frontal scalp, parietal scalp, and occipital scalp in a band-like pattern, as well as periocular and perioral, for the treatment of postmenopausal craniofacial hyperhidrosis. Three weeks after treatment, patients treated with BoNT-B reported a 91% improvement in the DLQI, compared with an 18% decrease in quality of life with placebo injections.31 In addition, BoNT injection has been shown to be clinically effective for the therapy of Frey’s syndrome and sialorrhoea; however given the anatomic location of injections, treatment is often performed by otolaryngology.32,33
In addition to hyperhidrosis, BoNT-A has been used successfully to treat chromhidrosis (coloured sweat of the axillae and cheek) and bromhidrosis (malodorous sweat of the axillae and genitals). Treatment protocols generally follow the same recommendations as for axillary hyperhidrosis, with patients receiving approximately 100 U, divided across the affected area(s), per treatment session. Clinical results last from 3 to 9 months.34,35 Furthermore, small studies demonstrate improvement in hand and dyshidrotic eczema (pompholyx) in patients with palmar hyperhidrosis after injection of 100 U of BoNT-A.36
Alopecia
BoNT-A has been used for the treatment of androgenetic alopecia, cephalalgic alopecia, alopecia areata, and radiation-induced alopecia. Although it is unknown how BoNT contributes to hair regrowth, it is hypothesised that decreasing microvascular pressure through muscle relaxation may increase oxygen delivery to the hair follicles. Treatment protocols range from 30 to 150 U injected into the frontal, temporal, periauricular, and occipital musculature over 1–12 sessions. Most studies report clinical improvement in hair growth or density and high levels of patient satisfaction; however, further randomised controlled trials are necessary to determine the true effect of BoNT on hair growth.37-39 On the contrary, multiple BoNT injections for forehead wrinkles have been associated with the development of frontal alopecia.40
Bullous skin disease
BoNT has been trialled off-label for the treatment of several bullous skin disorders including Hailey-Hailey disease (familial benign pemphigus), linear IgA bullous dermatosis, and localised epidermolysis bullosa simplex (Weber–Cockayne). Hailey-Hailey disease has been treated with BoNT-A injections, as well as a combination of BoNT-A and erbium: yttrium aluminum garnet ablative laser or oral tacrolimus, in the axillae, inframammary, groin and intergluteal cleft regions. Doses range from 25 to 200 U every 3 to 6 months with clinical improvement lasting at least 12 months after treatment.41,42 Case reports have reported the treatment of the bilateral axillae in a young patient with linear IgA bullous dermatosis using 50 U per axilla, and 100 U injected into the foot of a middle-aged female affected by localised epidermolysis bullosa simplex.43,44
Raynaud’s phenomenon
Raynaud’s phenomenon, vasospasm of the digits, is difficult to treat and is often recalcitrant to first-line therapies such as calcium channel blockers, nitrates, phosphodiesterase inhibitors, iloprost, and bosentan. Surgical procedures such as sympathectomy are invasive and require recovery and downtime. BoNT injection has been used successfully for the treatment of primary and sclerosis-associated Raynaud’s phenomenon of the fingers.45,46 After injection of 50–100 U of BoNT-A in 19 patients with Raynaud’s phenomenon, investigators noted that 13 patients reported immediate improvement in pain and chronic ulcerations healed within 60 days.47 When compared to normal saline injections, digital pulp temperature significantly improved in the fingertips treated with BoNT after 6 weeks, suggesting BoNT is effective at treating Raynaud’s phenomenon-associated vasospasm.48 Currently, there are no standardised injection protocols used for treatment; one study demonstrated that injection at the wrist, digits, or distal metacarpus does not result in significantly different clinical outcomes, although all were effective at treating Raynaud’s phenomenon-associated vasospasm.49
Scarring
BoNT has been used to decrease hypertrophic and keloidal scarring resulting from increased wound edge tension; by paralysing the surrounding musculature, tensile forces and abnormal scar formation may be decreased. It has also been suggested that BoNT may decrease TGF-β signalling, a regulator of hypertrophic scarring.50 Studies demonstrated the use of 15–140 U of BoNT-A administered up to 9 days post-procedure, every 4–12 weeks for 1–3 treatment sessions resulted in scar softening, reduced elevation, and decreased symptoms such as itching and pain.51 However, one study using a three-dimensional optical system to image keloidal scars, showed no keloidal tissue regression after treatment with BoNT.52 When compared to intralesional triamcinolone 10 mg/cc every 4 weeks for 6 injection sessions, 5 U/cm3 of BoNT-A every 8 weeks for 3 sessions was found to significantly improve subjective complaints such as itch, pain, and allodynia, possibly due to the clinical effects of BoNT-A on small-fibre neuropathy; however, only intralesional triamcinolone resulted in significant improvement in keloid scar softening.53
Combination therapy using BoNT-A has also been used for the treatment of hypertrophic or keloidal scarring. Clinical improvement of a traumatic scar of the chin was achieved with injection of 6–8 U of BoNT-A in combination with 4 treatments of 595 nm pulsed dye laser every 2 weeks.54 A combination of INCO and microneedling of facial scars after non-melanoma skin cancer surgery results in significantly improved cosmetic results and patient satisfaction with appearance post-surgery.55
Other medical diseases
Case reports have reported use of 40–250 U of BoNT-A per site (axilla, inguinal folds) with 1–3 treatment sessions for hidradenitis suppurativa, with complete remission of disease lasting 6 months to 3 years. Like bullous diseases, it is thought that decreased sweat production contributes to hidradenitis suppurativa improvement by eliminating an environment in which bacteria may flourish, and preventing rupture of the follicular unit.56,57 BoNT-A has also been trialled as an adjuvant for the treatment of aquagenic keratoderma,58 congenital eccrine naevus,53 Darier Disease (keratosis follicularis),59 inverse and plaque psoriasis,60 notalgia paraesthetica,61 pachyonychia congenita,62 and post-herpetic neuralgia resulting from herpes zoster.63
THE FUTURE
Multiple new formulations of BoNT-A are currently being trialled for the treatment of glabellar and periocular lines. DaxibotulinumtoxinA (DAXI; Revance Therapeutics Inc., Newark, New Jersey, USA) has been studied as both a topical and injectable therapy; but, the topical preparation was not efficacious. Injectable DAXI has entered Phase III FDA trials, with efficacy treating glabellar lines and clinical results possibly lasting 5 weeks longer than ONA (SAKURA 1 and 2, BELMONT).64 LetibotulinumtoxinA (LETI; Botulax®, Hugel, Chuncheon, South Korea) is currently commercially available in Asia and is undergoing studies in anticipation of FDA approval for treatment of periorbital rhytides.65 When compared to INCO, LETI has a greater amount of neurotoxin protein per volume, but also has increased amounts of inactive neurotoxin, which hypothetically may increase the risk of immunoreaction.66
Liquid formulations of BoNT-A are being studied to reduce current concerns for reconstitution contamination or error. NivobotulinumtoxinA (NIVO; Innotox®, Medytox, Inc., Seoul, South Korea, and MT10109L, Allergan, Inc., Irvine, California, USA) is non-inferior to ONA for the treatment of frown lines, and is undergoing studies to determine clinical efficacy for glabellar lines.67,68 QM-1114 (Galderma Laboratories, L.P., Lausanne, Switzerland) is superior for the treatment of glabellar lines compared to placebo, and is currently being studied for the reduction of lateral canthal lines.69-71
In addition to new formulations of BoNT-A, liquid BoNT-E (ED-001, Bonti, Inc., Newport Beach, California, USA) is being pursued due to its purported faster onset of action (24 hours post-injection) and decreased duration of clinical results (14–30 days). EB-001 has been shown to safely and effectively decrease appearance of glabellar lines and may improve forehead scar cosmesis post-Mohs micrographic surgery (SHINE).72,73 It is possible that in addition to the current cosmetic indications being pursued by pharmaceutical companies, dermatologists will be able to use these new BoNT-A formulations for the off-label treatment of dermatologic medical disease as outlined above.
CONCLUSION
BoNT is an extremely versatile injectable medication that can be used for cosmetic, as well as medical, treatment of dermatologic diseases such as hyperhidrosis, hair loss, aberrant scarring, bullous skin disorders, psoriasis, and Raynaud’s phenomenon, amongst others. Although the use of BoNT use is relatively safe, it is always important to be aware of injection points as the toxin may diffuse and negatively affect adjacent areas that should not have been treated. Clinicians should be acutely aware of site-specific adverse events, especially with injection of BoNT into the neck, hands, or feet. Dermatologists need to be educated on both the on- and off-label uses of BoNT to provide patients with appropriate treatment and decrease associated morbidity. Well-developed clinical trials are required to determine the true clinical efficacy of BoNT in the off-label setting, as well as possible long-term safety concerns.






