Abstract
Alopecia areata (AA) is a form of nonscarring alopecia, and is the most common immune-mediated cause of hair loss worldwide. Numerous therapeutic schedules available as off-label options have demonstrated only limited results. However, in 2022, baricitinib, a selective JAK1 and JAK2 inhibitor, was approved as an oral administered systemic therapy for severe AA. Based on this, the authors used it in a 21-year-old White female, who presented with a 15-year history of severe AA (Severity of Alopecia Tool score [SALT]: score 88) and immense psychological burden. After laboratory examinations within normal limits, baricitinib was administered as monotherapy with a 4 mg daily dosage. The severe AA improved rapidly after the first month, and resulted in total hair restoration just after the second month under baricitinib treatment. Besides clinical improvement, SALT score impressively reduced to 30 and 10, respectively, in 2 and 6 months. Six months later, the patient is keeping up the same treatment with no sign of relapse, and is on a 2-month follow-up schedule. In the authors’ patient, almost total hair restoration was achieved in less than 3 months of treatment, which strongly advocates for the addition of baricitinib in the dermatologic armament as a safe, adequate, and fast AA remedy.
![]()
Erratum: Baricitinib Demonstrates Rapid Action Within Just 2 Months of Treatment in Severe and Unresponsive Alopecia Areata: A Case Report
Authors: E. Tampouratzi, K. Sfaelos, M. Pizimola, P. Rigatos, J. Katsantonis
Original citation: Dermatol AMJ. 2024; DOI/10.33590/dermatolamj/10307342. https://doi.org/10.33590/dermatolamj/10307342.
Date correction published: 04.23.24
The article by Tampouratzi et al. in AMJ Dermatology 1.1 (pages 63-67) was originally published on 03.25.25. Since then an erratum has been made. The data points in Figure 2 were originally presented incorrectly. This has now been updated.
The AMJ apologizes for the error and any inconvenience caused.
![]()
Key Points
1. Alopecia areata (AA) has an extremely negative impact on a patient’s quality of life, especially young patients, making the need for new treatments such as baricitinib imperative.2. Daily clinical practice improves response in severe AA, with total hair restoration achieved in less than 3 months of treatment.
3. JAK inhibitors may represent the drug of choice for AA, as they have revolutionized the therapeutic outcome of this devastating disease.
INTRODUCTION
Among the different types of alopecia, alopecia areata (AA) is the most common reason of hair loss worldwide that is attributed to an immune-mediated process.1,2 The documented AA global incidence is within the spectrum of 0.57–3.80% from hospital-based studies, and 1.7–2.1% from general population studies that attempt to calculate the lifetime incidence of AA.3 A total of 10–20% of people who are affected by AA have a positive family history of the disease, indicating a strong genetic background.4 Atopic dermatitis, vitiligo, and other relevant autoimmune disorders, such as systemic lupus erythematosus, are linked with AA, indicating common, but to this day unidentified, pathogenic mechanisms.5,6 AA can affect any region that bears hair, and can present in a wide spectrum that can range from patchy diffuse alopecia to alopecia totalis or universalis.7 The disease impacts quality of life (QoL) with major psychological effects, such as self-esteem issues, depression, and social anxiety, both in males and females.8,9
There are several therapeutic options available, including off-label ones (corticosteroids, cyclosporine, contact immunotherapy, hydroxychloroquine), but these have demonstrated only limited results, and highlight the autoimmune nature of AA.10,11,12 However, in 2022, regulatory authorities approved baricitinib, a selective JAK1 and JAK2 inhibitor, as per os systemic treatment for severe AA.13 Two pivotal randomized, placebo-controlled, Phase III clinical trials showed good clinical efficacy in severe disease versus the placebo treatment arm, with regard to hair regrowth at the 36 weeks timepoint, with favorable safety profile.14 Based on these results, the authors administered baricitinib in a female patient of young age suffering from severe AA, who presented inadequate response to previous applied treatment modalities, with excellent and quick response in less than 3 months of treatment.
PATIENT INFORMATION
A 21-year-old White female patient presented with a 15-year history of severe AA and immense psychological burden. The severity of disease was assessed with the Severity of Alopecia Tool (SALT) score, with an initial value of 88 in this patient.15,16 Regarding the impact on the patient’s QoL, the Dermatology Life Quality Index (DLQI) questionnaire was utilized, scoring 21 during baseline evaluation. An informed consent form was obtained from the patient. No ethical approval was necessary for the specific case report.
CLINICAL FINDINGS
A generalized hair loss in round and oval areas with no apparent inflammation was noticed across the entire scalp, on the vertex area of the head, and in the parietal and occipital regions, as shown in Figure 1.
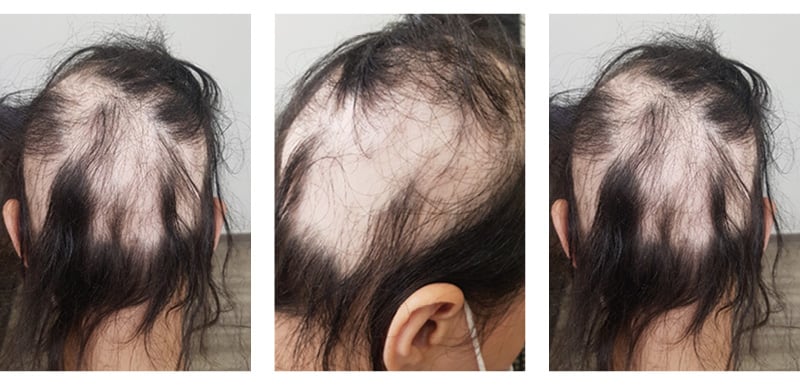
Figure 1: Patient with severe alopecia areata before treatment.
TIMELINE
The patient had a strong genetic predisposition, with a positive family history of AA from her mother, and onset of the disorder in her brother as well. The rest of her medical history was free of other systemic diseases, except for the severe emotional burden of AA, and a baseline DLQI score of 36. Prior to her visit, she had consulted many dermatologists, and had been exposed to various treatment modalities, including topical, intra-lesional, and oral corticosteroids; contact immunotherapy with diphenylcyclopropenone; topical minoxidil; cystine vitamin; and other immunosuppressive agents like cyclosporine that were proven insufficient, further worsening her overall QoL.
DIAGNOSTIC ASSESSMENT
The patient underwent a complete laboratory control (full blood count with differential comprehensive metabolic panel, lipid profile after fasting, along with HIV, tuberculosis, and hepatitis B and C screening), before initiating treatment on oral JAK inhibitor baricitinib. The drug was administered as monotherapy in a 4 mg daily dosing scheme.
THERAPEUTIC INTERVENTION AND FOLLOW-UP OUTCOMES
Signs and symptoms of severe AA were rapidly improved after the first month (SALT score: 30), leading to total hair restoration after 2 months on baricitinib treatment (SALT score: 10). Six months following initiation of treatment, the patient is maintaining the same treatment without any sign of relapse. In addition, the laboratory results are within normal limits at the 6-month follow-up evaluation. The patient’s SALT score and clinical status at 6-month follow-up are indicated in Figures 2 and 3. Regarding the impact of treatment from a QoL perspective, DLQI assessment provided a score of 5 and then 0, at 1-month and 6-month evaluation, respectively. The patient is still on a 4 mg daily dosing scheme, and she is continually on a 2-month re-evaluation schedule in order to monitor progress of treatment.
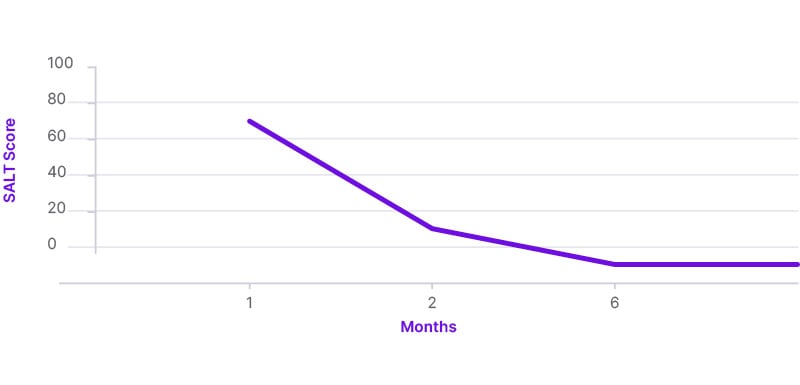
Figure 2: Severity of Alopecia Tool (SALT) score showing the improvement of alopecia areata during the 6-month follow-up.
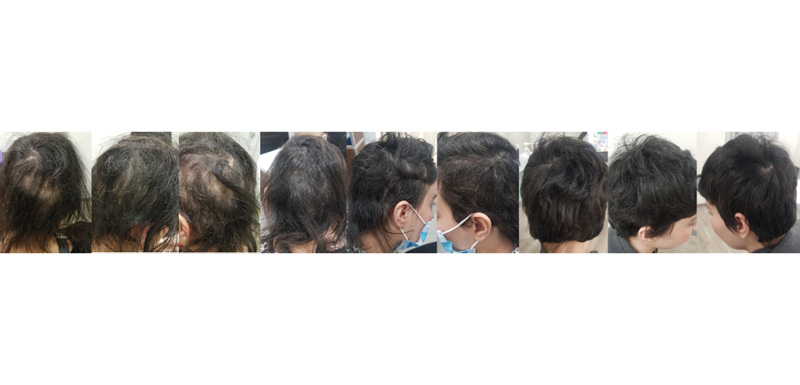
Figure 3: Hair regrowth after 1 month, 2 months, and 6 months, following treatment initiation with baricitinib.
DISCUSSION
Baricitinib, a first generation oral JAK inhibitor, with a mode of action that inhibits JAK1, JAK2, and to a lesser extent JAK3, interrupts the cytokine signaling pathways that have a key role in the pathogenesis of AA.17
Ιn the pivotal randomized, placebo-controlled, Phase III clinical trials BRAVE-AA 1 and BRAVE-AA 2, 38.9% of enrolled patients in the first study and 35.9% in the second achieved a SALT score of 20 or less at Week 36 under baricitinib treatment at a 4 mg dosage.14 In the authors’ case study, the patient achieved a SALT score of 30 in 4 weeks, and 10 in 8 weeks on the same dosage.
Another systematic review and meta-analysis, which included data from five randomized studies regarding patients with AA that were defined as >50% scalp hair loss, treated with baricitinib 4 mg once per day, presented the highest efficacy compared to other oral JAK inhibitors (ruxolitinib, tofacitinib, ritlecitinib, brepocitinib, and delgocitinib).12,18-21
Besides clinical trials, systematic reviews, and case study series, isolated case reports, such as Jabbari et al.’s22 from 2015, reported complete hair regrowth after 9 months of treatment with baricitinib on an 11 mg daily dosing scheme. Similar findings were observed by Olamiju et al.23 in 2019, with almost complete hair regrowth after 8 months of therapy with baricitinib 4 mg daily. Finally, Wang et al.,24 in 2022, observed response to baricitinib therapy at 5 months, which is the shortest report available to date.24
In addition to efficacy, safety data are already supported since 2011, by Shi et al.,25 who observed that per os administration of baricitinib in healthy controls either produced no or mild adverse effects (reduced reticulocyte count and neutropenia).25 There were no malignancy cases reported in relation to baricitinib use. Based on findings from a study that assessed potential carcinogenicity in mice models, baricitinib was found to have no carcinogenic effect.26 Furthermore, in BRAVE-AA 1 and BRAVE-AA 2 trials, in 52 weeks of follow-up, there were no new safety signals compared to the short-term results.27 The rates of side effects with baricitinib generally reflect the inherent risk of the disease populations being treated, and are found more often in rheumatic patients who have an burden of disease.28-30 Additionally, imaging techniques such as line-field confocal optical coherence tomography can be used to observe and monitor early, subclinical hair regrowth during treatment with baricitinib, as has been recently demonstrated by Verzì et al.31
As elucidated by reviewing the above available references, the authors reasonably suggest that we could anticipate even faster response to baricitinib for severe AA cases. The authors’ patient achieved almost total hair restoration within 3 months of treatment, despite the fact that she had AA for more than 8 years without any hair regrowth during that time period. This strongly supports the addition of the specific treatment option in the dermatologic armament as a safe, adequate, and fast AA remedy.






