Epidermolysis bullosa acquisita (EBA) is a rare, sub-epithelial, mechano-bullous blistering disease that usually develops in adulthood. Treatment of EBA with conventional immunosuppressive agents is generally unsatisfactory. At the 26th congress of the European Academy of Dermatology and Venereology (EADV) in Geneva, we presented a case of EBA successfully treated with a rituximab and high-dose intravenous immunoglobulin (IVIg) combination.
A 52-year-old female with a 12-year history of EBA presented with widespread vesiculobullous eruption and milia formation on the trunk and extensor surface of the extremities and scalp accompanied with pruritus (Figure 1). She was unresponsive to prednisolone and azathioprine; therefore, a combination of rituximab (two doses of 1,000 mg, 15 days apart) and human Ig (0.4 g/kg 5 days per month) was added to treatment. At 6-month follow-up, lesions on the extremities had improved almost completely (Figure 2).
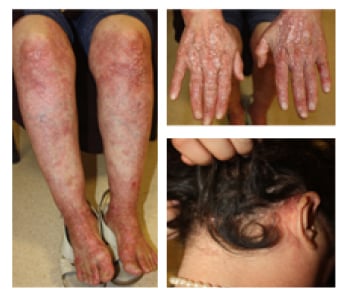
Figure 1: Widespread milia formation on the lower extremities, hands, and scalp.
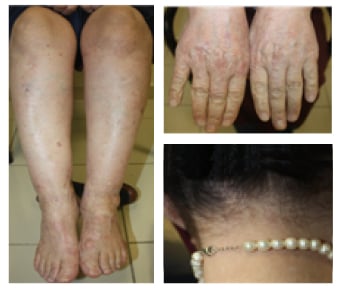
Figure 2: Clearance of widespread milia lesions on the lower extremities, hands, and scalp after rituximab–IVIg combination treatment.
IVIg: intravenous immunoglobulin.
Compared with other autoimmune blistering diseases, EBA is generally resistant to conventional treatments; remission induction is quite difficult with the available options such as systemic corticosteroids and immunosuppressive agents. In recent years, much more experience has been gained in the treatment of autoimmune bullous disease with the emergence of novel therapeutic agents. Rituximab and IVIg are new treatment options that can prolong the disease-free interval in patients with an autoimmune bullous disease and have been increasingly used. Rituximab, a monoclonal anti-CD20 antibody targeting immature B cells and memory B cells, can be administered as a monotherapy or in combination with adjuvant agents. IVIg can reduce pathogenic autoantibody levels, and long-term remission can be achieved using IVIg monotherapy in patients with autoimmune bullous diseases. The rituximab–IVIg combination can decrease levels of pathogenic autoantibodies rapidly. A further advantage of this combination therapy is its ability to reduce the risk of infection due to immunosuppression caused by rituximab. The combination of rituximab–IVIg was reported to produce clinical remission in a small number of patients with EBA, which was sustained during the follow-up period. It is suggested that combined/single use of biological agents, such as IVIg and rituximab, is an effective first-line treatment and may protect against the side effects of systemic corticosteroids and immunosuppressive agents.
In conclusion, rituximab–IVIg combination treatment is effective and safe for treating patients with EBA resistant to conventional treatments. IVIg therapy rapidly removes blood pathogenic autoantibodies and reduces the possible risk of infection caused by rituximab. In addition, pathogenic antibody levels can be reduced in the long term by changing the B cell subgroups with rituximab.







