BACKGROUND AND AIMS
Psoriasis is a chronic inflammatory skin disorder with systemic implications, and a range of comorbidities, including psoriatic arthritis, cardiovascular disease, and obesity.1 Advances in our understanding of its pathogenesis have led to the development of highly effective therapies that target the IL-23/T helper 17 pathway.2 Patients with moderate-to-severe plaque psoriasis often require systemic treatments, such as biologics. Brodalumab, a fully human recombinant monoclonal antibody of the IgG2 type, binds strongly to IL-17 receptor A,3 effectively inhibiting pro-inflammatory cytokines like IL-17A, IL-17F, IL-17A/F, IL-17C, and IL-17E. This mechanism of action helps alleviate inflammation and associated clinical symptoms in patients with psoriasis. The primary objectives of this analysis were to assess the efficacy and safety of brodalumab at the University Hospital Joan XXIII of Tarragona, Spain, and compare these findings with those from clinical trials.4
MATERIALS AND METHODS
The study included 31 patients with moderate-to-severe plaque psoriasis who had received treatment with brodalumab for at least 36 weeks, with a maximum follow-up of 52 weeks. Efficacy and quality of life were evaluated using Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores at Weeks 4, 12, 24, 36, and 52. Of the patients, 83.9% (n=25) had previously received only one biological therapy.
RESULTS
A rapid improvement in PASI scores was observed (Figure 1A), with a 66% reduction from baseline at Week 4, and 90% at Week 12. By Week 12, 84%, 68%, and 58% of patients achieved PASI 75, 90, and 100, respectively. This improvement was sustained until Week 52. Quality of life also improved rapidly (Figure 1B). A DLQI score reduction of 82% was noted at 4 weeks, reaching 93% at Week 12, and maintaining this improvement until Week 52. Six patients discontinued treatment due to lack of efficacy, three achieved disease remission at Week 36, and one stopped treatment due to the appearance of joint involvement. Efficacy data, as assessed by PASI scores, were analysed based on BMI and the number of prior treatments received before starting brodalumab (Figure 1C). No significant differences were observed in terms of efficacy or rapid response among different BMI or previous treatment line groups.
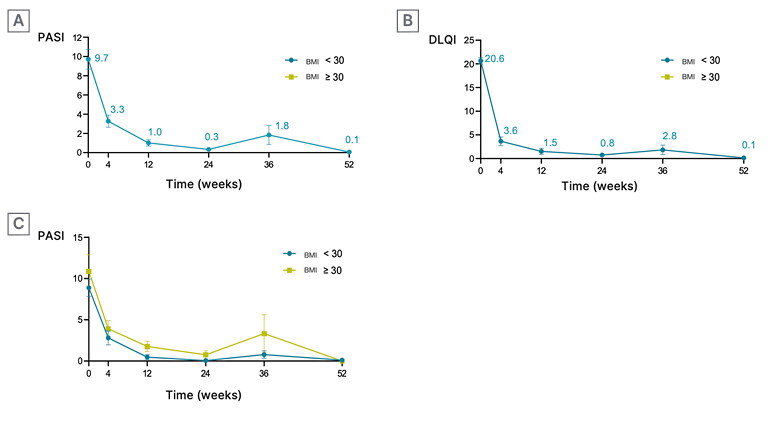
Figure 1: Evolution of efficacy and quality of life (using Psoriasis Area and Severity Index and Dermatology Life Quality Index scores) over time.
A) Rapid improvement in PASI: PASI score evolution over time, measured in weeks.
B) Rapid improvement in quality of life: DLQI score evolution over time, measured in weeks.
C) Utility in different patient cohorts: PASI score evolution over time, measured in weeks, in patients with BMI <30 kg/m2 and BMI >30 kg/m2.
DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index.
CONCLUSION
In this case series, brodalumab demonstrated high effectiveness and a rapid onset of action, with a 90% reduction in baseline PASI scores by Week 12. These results are consistent with those observed in the AMAGINE-2 and AMAGINE-3 clinical trials.4 Concurrent improvements in quality of life were also evident. Notably, in this case series, most patients had obesity, suggesting that brodalumab is an effective treatment option regardless of BMI. Regarding the number of previous treatment lines, no definitive conclusions on efficacy could be drawn, given that most patients had only received one prior treatment. Importantly, there was only one discontinuation due to adverse effects, indicating a favourable safety profile.






