INTRODUCTION
Alopecia areata (AA) is a condition that causes non-scarring hair loss; the estimated lifetime risk of developing the disorder is 1.7% but the aetiology of AA is still being investigated. AA has an autoimmune background, but is also related to various atopic and autoimmune disorders, such as vitiligo and thyroid disease.1-3 The course of the disease is unpredictable, but it is known that early age of onset, extensive hair loss, nail changes, and comorbid autoimmune disorders are associated with a poor outcome. The disease also affects patient quality of life, as treatment of AA is often unsatisfactory. Topical or intralesional corticosteroids are used as the first-line therapy for AA. It has been demonstrated that topical immunotherapy with diphenylcyclopropenone (DPCP) is a good therapeutic option for patients with AA; DPCP is a contact allergen, and the effectiveness of the drug has been demonstrated in several reports.4,5
MATERIALS AND METHODS
A total of 106 (70 female and 36 male) patients with AA were enrolled in our study. The mean age of the female patients was 38.4±13.3 years and male patients 30.9±12.0 years; the patients’ average disease duration was 107.1±133.6 months. All patients underwent a standardised diagnostic protocol, which included the collection of clinical and demographic data and the evaluation of the severity of the disease with the Severity of Alopecia Tool (SALT). We divided SALT scores into five groups depending on percentage of hair loss: S1: ≤24%, S2: >24–49%, S3: >49–74%, S4: >74–99%, and S5: 100% of hair loss.
Hair regrowth after 6 months of treatment was calculated according to the formula: [(A-B)/A]x100%, where A is the percentage of hair loss before treatment and B is the percentage of hair loss after 6 months of treatment.6 Treatment was performed with a 1×10-6–2% DPCP solution. After the first application of the DPCP solution, the DPCP concentration was slowly increased to the maximum concentration that was acceptable for the patients and was adjusted to clinical response. We also assessed quality of life using the Dermatology Life Quality Index (DLQI) before treatment and after 6 months of treatment.
RESULTS
The mean percentage of hair loss before treatment was 51.0±35.1% and after 6 months of treatment, the percentage was 43.6±40.7%. Women had more episodes of AA from the beginning of the disease and higher prevalence of thyroid disease than men (p=0.003). We found that the severity of hair loss did not correlate with thyroid disease or atopy (p>0.05). Moreover, the presence of thyroid disease did not affect hair regrowth (p>0.05). We found a correlation between severity of the disease (according to SALT scores) before and after treatment (p<0.001). The best results of treatment were observed in S3 and S4 group patients (Figure 1). Quality of life measured before treatment ranged from 0–24 points, with a mean score of 5.5±5.2 points, and after 6 months of treatment the mean value of DLQI was 3.2±5.1 points. We found that the DLQI scores of AA patients were significantly lower after 6 months of treatment (rho=0.376; p<0.05).
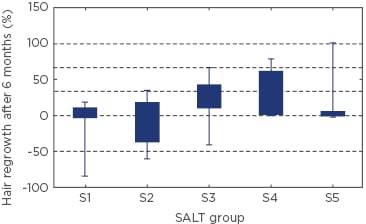
Figure 1: Hair regrowth in patients with various severities of alopecia areata (SALT S1-S5).
Data were analysed using the Kruskal–Wallis test. H(4;75)=9.41; p=0.051.
S1: ≤24% hair loss, S2: 25–49% hair loss, S3: 50–74% hair loss, S4: 75%– 99% hair loss, and S5: 100% hair loss; SALT: Severity of Alopecia Tool.
CONCLUSION
Treatment of AA with DPCP led to a decrease in disease severity. Topical immunotherapy is an effective treatment for AA and improves quality of life.







