BACKGROUND AND AIM
Four patients with long-standing atopic dermatitis (AD) with elevated Eczema Area and Severity Index (EASI) and nonresponders to common therapies have been treated with the innovative drug for AD, dupilumab. Dupilumab is a fully human monoclonal IgG4 antibody targeting the α-subunit of the IL-4 receptor (IL-4Rα).1-4 IL-4Rα is a part of Type I and Type II IL-4 receptors and the IL-13 receptor; therefore, blocking it inhibits the downstream signaling of IL-4 and IL-13. IL-4 and IL-13 are both crucial cytokines of the Th2 pathway. The aim of the study was to bring the authors’ experience with this new drug to patients from southern Italy.
MATERIALS AND METHODS
The study involved 4 patients, the male to female ratio was 1:1, and the mean age was 36.25 years.
All the patients had AD from infantile age that had been treated over time with topical corticosteroids, topical calcineurin inhibitors, and also oral cyclosporine without significant improvement of the pathology. One patient had to stop cyclosporine because of increased blood pressure. All patients had AD in severe form with dry skin, and red-to-brownish patches on the hands, ankles, wrists, and neck with lichenification of the folds. One patient presented with pityriasis alba in face and limbs with important psychological implications on his social life. To evaluate the effectiveness of dupilumab, the authors used the following scores: EASI, itch-visual analogue scale (VAS), and dermatology life quality index (DLQI). The patients were revalued after 3 months of therapy (T1) with iconographic documentation.
RESULTS
Significantly positive results of the scores were obtained at 3 months of observation without any adverse effect (Table 1). All patients showed rapid clinical improvement of cutaneous lesions and no other new lesions were reported during treatment. From T0 to T1, EASI showed a reduction of -7.7% in the first patient, -78.4% in the second patient, -100.0% in the third patient, and -92.6% in the fourth patient. The average value for the reduction of EASI in all patients was 89.675. Reduction of daily itching was reported after the first month of treatment. From T0 to T1, VAS showed a reduction of -100.0% in the first patient, -88.9% in the second patient, -100.0% in the third patient, and -90.0% in the fourth patient. The average value in the reduction of VAS of all patients was 94.725. All aspects of AD regressed; the quality of life improved, as can be seen from the value of DLQI; and patients had fewer problems dealing with others and in their manual and work activities. From T0 to T1, DLQI showed a reduction of -100.0% in the first patient, -85.0% in the second patient, -92.3% in the third patient, and -100.0% in the fourth patient. In the average value of all patients the reduction of DLQI was 94.325.
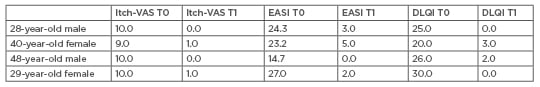
Table 1: Quantitative indexes score.
DLQI: dermatology life quality index; EASI: eczema area and severity index; Itch-VAS: itch-visual analogue scale; T0: before treatment; T1: after 3 months of therapy.
CONCLUSIONS
The data obtained show that the biologic drug for AD notably improves the patients quality of life, acting initially on the itching, and subsequently on the clinical picture of the disease opening a new era in the treatment of AD in chronic and severe patients.







