Meeting Summary
This symposium brought together experts in cardiology, nephrology, diabetology, and clinical pharmacology to discuss best practice when caring for patients with atrial fibrillation (AF) and comorbidities. They urged delegates to not only consider the issue of AF but also to think about protection in a broader sense, including comorbidities to improve outcomes for patients when it comes to stroke prevention. Dr Ruff spoke of the tremendous opportunity to reduce the burden of stroke by addressing important modifiable risk factors for stroke, focussing on AF and diabetes, and their link to chronic kidney disease (CKD). Dr Bonnemeier and Dr Kreutz discussed patients with AF and renal dysfunction, noting that CKD is a frequent comorbidity associated with increased risk of stroke and bleeding among patients with AF. The associated patient case study inspired debate about the challenges of oral anticoagulant (OAC) therapy in this patient group and highlighted that while decline in renal function is common in AF patients treated with OAC, the extent of decline may depend on which anticoagulant is used. Furthermore, available data from randomised control trials and recent retrospective analyses were shared which showed differences in the progression of CKD associated with vitamin K antagonists (VKA) versus the novel OAC (NOAC), such as rivaroxaban. Dr Patel and Dr Rossing focussed on diabetes and AF, stating that their frequent coexistence is a bad combination associated with substantially increased risks of death and cardiovascular (CV) events. Exploring the link between diabetes and CKD, they demonstrated the significant impact renal dysfunction has on the prognosis of Type 2 diabetes mellitus (T2DM). They additionally presented recent evidence from retrospective analyses comparing renal outcomes in patients with AF and diabetes treated with NOAC or VKA, noting that choice of anticoagulation may impact risk for renal outcomes.
Introduction: What is the Bigger Picture?
Doctor Gilbert Deray and Doctor Christian Ruff
Dr Deray introduced a multidisciplinary panel of speakers from cardiology, nephrology, diabetology, and clinical pharmacology, providing a comprehensive look at how to improve outcomes in patients with AF and comorbidities, striving for protection beyond stroke risk. While tremendous advances in both stroke prevention and treatment have improved patient outcomes, stroke remains the second most common cause of death globally after ischaemic heart disease; yet up to 80% of strokes can be avoided.1
Dr Ruff acknowledged that physicians are providing anticoagulants to AF patients to prevent strokes but questioned if more could be done to protect patients with comorbidities. He pointed out that approximately 90% of the population-attributable risk factors of stroke are caused by potentially modifiable risk factors,2 and stated that this provides a “tremendous opportunity” to greatly reduce the stroke burden around the world.
Explaining that the panel would focus on two of these modifiable risk factors, diabetes and AF (including renal dysfunction), Dr Ruff noted that AF is associated with a 5-fold increase in risk of stroke3 and diabetes, and with a 2-fold increase in the risk of stroke.4 Furthermore, diabetes is a risk factor for AF and a common cause of CKD, which is associated with a 30–60% increase in ischaemic stroke (IS) risk.5
Dr Ruff stated that the reason for focussing on prevention of AF-associated stroke is because the related outcomes are worse than for non-AF strokes.6 One in four patients that are admitted with IS associated with AF will die within 30 days, making AF stroke almost twice as likely to be fatal than non-AF stroke. Furthermore, 30% of the patients who survive an AF-related stroke have severe dependence at 12 months compared with 11% for non-AF stroke.6
Registry data suggest that the use of OAC therapy remains suboptimal across the world,7 and according to Dr Ruff, physicians are still on a journey to optimise the therapies available to better protect patients when it comes to stroke prevention. He added: “We need to take a step back and look at the bigger picture and investigate the comorbidities and complex patients we see in practice.”
Think about the Kidneys: Why does Renal Function Matter in Patients with Atrial Fibrillation?
Doctor Hendrik Bonnemeier and Doctor Reinhold Kreutz
Reiterating the need to look beyond AF, Dr Bonnemeier described how he sees AF patients with comorbidities such as arterial hypertension, coronary artery disease (CAD), obesity, diabetes, and CKD, and noted that these diseases overlap and interact. He outlined a ‘typical’ patient case, a 66-year-old male presenting with palpitations.
The patient had arterial hypertension (blood pressure: 150/90 mmHg), diabetes (receiving dietetic therapy), and was overweight (BMI: 29). He had persistent nonvalvular AF (NVAF) and had undergone external cardioversion twice. His electrocardiogram showed AF with a heart rate of around 100 beats per minute.
After around 20 hours, the patient spontaneously converted into sinus rhythm. He had undergone a heart procedure 2 years before, with exclusion of significant CAD, and echocardiography revealed good left ventricle function and left ventricular hypertrophy as a result of hypertension.
The patient was on rivaroxaban 20 mg once daily (od), verapamil 120 mg twice daily, ramipril 5 mg twice daily, torasemide 5 mg od, and pantoprazole 20 mg od.
The lab findings showed creatinine 1.89 mg/dL, estimated glomerular filtration rate (eGFR) 46 mL/min, mild increase in c-Troponin T (0.2 ng/mL), and mild increase in N-terminal pro-B-type natriuretic peptide (198 pg/mL), probably due to the AF.
Dr Bonnemeier and Dr Kreutz agreed that there are several issues to consider when thinking about anticoagulation to manage thromboembolic risk in a multimorbid patient such as this case, not least the impact of CKD. They noted several issues to consider in patients with AF and CKD including the need to balance the risks of both IS and bleeding, the need to monitor renal function and to select appropriate dosing based on the level of renal function, and how choice of anticoagulant therapy can affect renal outcomes.
The 2018 European Heart Rhythm Association (EHRA) Practical Guide on the use of NOAC in patients with AF advises on the optimal use of NOAC according to renal function.8 Dr Kreutz outlined the guidance for rivaroxaban, noting that it is evidence based9 and straightforward: 20 mg od for patients with a creatinine clearance (CrCl) of >50 mL/min, and 15 mg od for patients with a CrCl of 30–49 mL/min. Cautionary use of 15 mg od is recommended for patients with severe renal impairment (CrCl: 15–29 mL/min) and in Europe, no NOAC is recommended for patients with end stage renal disease (ESRD; CrCl: <15 mL/min) undergoing dialysis.
The Phase III ROCKET AF trial, which compared the efficacy and safety of rivaroxaban to warfarin in 14,264 AF patients, studied a specific renal dose of rivaroxaban to support safety, and 1,474 patients with moderate renal impairment received the reduced dose of 15 mg od.9
Atrial Fibrillation and Chronic Kidney Disease: Knowing the Risks
CKD is a frequent comorbidity in patients with AF and is associated with adverse outcomes.10,11 A large Danish cohort study (N=132,372) showed that CKD was associated with an increased risk of stroke or systemic thromboembolism (SE) and bleeding among patients with AF.11
Dr Kreutz touched upon the interaction between vascular calcification and CKD, noting that medial vascular calcification is highly prevalent in patients with CKD.12 Research suggests that vascular calcification affecting the kidneys is a possible side effect of VKA treatment. It is further hypothesised that VKA, such as warfarin, promote vascular calcification because the effect of VKA is not limited to coagulation, but affects all vitamin K-dependent proteins including matrix G1 protein, which plays a major inhibitory role in the development of vascular calcification.13 Dr Kreutz suggested that treatment with a VKA, which inhibits the activation of matrix G1 protein and thereby abolishes its protective effect against calcification, may contribute to worsening renal function and accelerate progression of kidney disease.
Supporting this concept, post-trial analyses of the RE-LY (comparing the efficacy and safety of dabigatran to warfarin) and ROCKET AF trials showed that AF patients treated with warfarin had a significantly greater decline in renal function over the course of the study compared to the NOAC arms.14,15
Differences in Progression of Chronic Kidney Disease
A retrospective analysis of a large USA administrative database suggested decline in renal function is common in AF patients treated with OAC, but the extent may depend on which anticoagulant is used. It found NOAC, including rivaroxaban, were associated with lower risks of adverse renal outcomes over time compared to warfarin. The study compared three NOAC (apixaban, dabigatran, and rivaroxaban) to warfarin for their effects on four renal outcomes: >30% decline in eGFR, doubling of the serum creatinine level, acute kidney injury (AKI), and kidney failure. When comparing each NOAC with warfarin, rivaroxaban was associated with lower risks of >30% decline in eGFR, doubling of serum creatinine, and AKI; dabigatran was associated with lower risks of >30% decline in eGFR and AKI; however, apixaban did not have a statistically significant relationship with any of the renal outcomes.16
Recent real-world data from a subgroup analysis of the retrospective cohort study RELOAD, which compared the effectiveness and safety of rivaroxaban to phenprocoumon (a VKA widely used in Germany) in patients with NVAF and renal impairment, showed that when using the ‘one tablet per day’ definition of estimating drug exposure time, the incidence of the primary endpoint of IS was significantly lower in patients (without evidence of cancer at baseline) receiving rivaroxaban 15 mg or 20 mg od compared with those receiving phenprocoumon (2.40 versus 3.51 events per 100 patient-years, respectively; p=0.015). There was also a trend towards lower risk of the primary safety outcome of intracranial haemorrhage (ICH) for rivaroxaban versus phenprocoumon (0.57 versus 0.89 events per 100 patient-years; p=0.14).17
Furthermore, new findings from RELOADeD, an observational study in the European Union (EU), comparing rivaroxaban, apixaban, and edoxaban to phenprocoumon in patients with NVAF and renal disease revealed a comparable risk of IS/SE for all NOAC compared to phenprocoumon, and a beneficial effect for both rivaroxaban and apixaban with regards to ICH. Results showed significant risk reductions related to ESRD/dialysis for rivaroxaban (73%) and apixaban (57%) compared to phenprocoumon, while for the risk of AKI, this trend was only seen for rivaroxaban (Figure 1).18
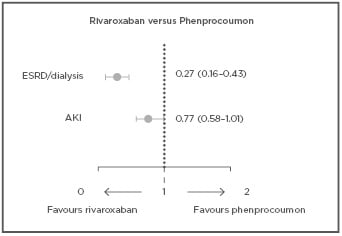
Figure 1: Confounder-adjusted hazard ratios of renal safety outcomes with 95% confidence intervals for rivaroxaban versus phenprocoumon in patients with NVAF and renal disease.
A multiple Cox-regression was performed to calculate confounder-adjusted hazard ratios for the risk of ESRD and AKI in new users of NOAC versus new users of phenprocoumon. Results indicated a beneficial effect of NOAC in renal function worsening over time when compared to phenprocoumon in patients with NVAF and renal disease.18
AF: atrial fibrillation; AKI: acute kidney injury; ESRD: end-stage renal disease.
Adapted from Bonnemeier et al.18
Rivaroxaban was also associated with lower risk of AKI or progression to Stage 5 CKD compared with warfarin in the RIVAL study, which used USA Truven MarketScan claims data to compare the impact on renal outcomes in NVAF patients (Stage 5 CKD or haemodialysis excluded). Rivaroxaban was additionally associated with a 19% risk reduction in AKI and an 18% reduction in progression to Stage 5 CKD or haemodialysis compared to warfarin.19
To further investigate the observed lower risks of renal adverse events with rivaroxaban compared to VKA, the prospective XARENO (Factor XA -inhibition in RENal patients with non-valvular atrial fibrillation Observational registry) study is ongoing. The multicentre study will collect data from >2,500 patients with NVAF and eGFR/CrCl 15–49 mL/min and compare progression of CKD and clinical outcomes in patients receiving rivaroxaban, VKA, or no anticoagulation therapy for >3 months. The first results are expected at the end of 2020.20
Panel Discussion Highlights
- Delegates and the panel discussed the lack of clear evidence on the efficacy and safety of NOAC in patients with ESRD or on dialysis, and the need for further studies, noting that the use of NOAC in patients with severe renal function impairment (CrCl <15 mL/min) or those on dialysis is not recommended by the EHRA Guidelines, nor by the respective EU labels for each drug; dabigatran is contraindicated in CrCl <30 mL/min.
- The panel suggested that helping to reduce the need for dialysis through preservation of renal function was critical and stated this is a “key point” when making decisions about anticoagulation.
Think About Diabetes: More than just a Thromboembolic Risk Factor in Patients with Atrial Fibrillation?
Doctor Manesh Patel and Doctor Peter Rossing
Dr Patel began by sharing a patient case study of a 68-year-old female with AF, hypertension, and diabetes, describing diabetes as “the 21st century plague.”
- The patient had some peripheral neuropathy and her family was concerned about some unsteadiness. She also experienced pain in her legs when walking, but it is unclear if this was because of peripheral neuropathy or peripheral arterial disease (PAD).
- She denied any congestive heart failure symptoms and upon examination had chronic AF with a heart rate of 73 beats per minute and an eGFR of 43 mL/min.
- Current medications include metformin, amlodipine, atorvastatin, and multivitamins.
- The patient and her family were interested in determining if she should be on an OAC.
Dr Patel handed over to diabetologist Dr Rossing to discuss his thoughts on the presented case. After thanking delegates for having a diabetologist at the European Society of Cardiology (ESC) meeting, Dr Rossing said this patient case illustrated a big overlap between diabetes and cardiology, and also with CKD because a significant percentage of patients with diabetes have CKD.21 The United States Renal Data System (USRDS) 2017 report showed that among National Health and Nutrition Examination Survey (NHANES) participants with diabetes, 28.7% had increased albuminuria, 20.7% had impaired renal function, and 10.0% had both.21
Diabetes and hypertension are the most common causes of CKD.22 Dr Rossing explained that diabetes and hypertension increase the risk of kidney disease through a variety of pathways, including inappropriate activation of the renin–angiotensin–aldosterone system, impaired insulin-mediated vasodilatation, augmented sympathetic nervous system activation, altered innate and adaptive immunity, and abnormal sodium processing by the kidney.23 Kidney disease has a significant impact on the prognosis of T2DM. Patients with T2DM and albuminuria or impaired renal function had an increased risk of mortality compared with T2DM patients with healthy kidneys, and the risk was further increased in patients with both albuminuria or impaired renal function.24
Diabetes also increases the risk of developing AF.25,26 The Framingham Heart Study showed that having diabetes increased the odds of developing AF by 40% for men and 60% for women.25 In another large cohort study, diabetes was identified as a strong independent risk factor for AF.26 Dr Rossing noted that AF and T2DM frequently coexist and described them as a “bad combination” associated with substantially increased risks of death and CV events.27 The large ADVANCE study including 11,140 patients with T2DM, of whom 7.6% had AF at baseline, showed that AF is associated with 61% greater risk of all-cause mortality and 68% increased risk of major cerebrovascular events in patients with diabetes.27 He said physicians taking care of patients with diabetes and CKD need to look out for AF, and screen and intervene not only for glucose but all the relevant risk factors in this population.
Less Risk of Renal Adverse Events
The RELOADeD study was revisited, with a focus on patients with NVAF and diabetes initiating rivaroxaban, apixaban, edoxaban, or phenprocoumon. Dr Patel shared recent results indicating the NOAC, particularly rivaroxaban and apixaban, are associated with less renal adverse effects over time compared to phenprocoumon. A comparable risk of IS/SE was seen for each NOAC compared to phenprocoumon, with a trend towards better effectiveness for rivaroxaban. There was a numerical benefit for NOAC over phenprocoumon for the risk of ICH and significant risk reductions related to ESRD for rivaroxaban (68%) and apixaban (40%). For the risk of AKI, only rivaroxaban showed a risk reduction (28%).28
Furthermore, recent findings from a retrospective analysis of USA claims data for patients with NVAF and diabetes also suggest that rivaroxaban is associated with lower risks of renal adverse effects than warfarin. Rivaroxaban was associated with a 17% lower risk of AKI and an 18% lower risk of progression to Stage 5 CKD or haemodialysis compared to warfarin (Figure 2).29
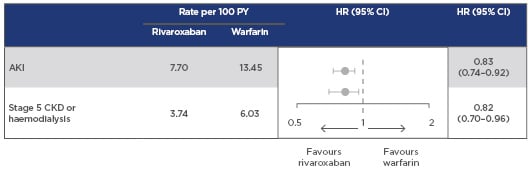
Figure 2: Risk of major adverse renal outcomes with rivaroxaban versus warfarin.
Retrospective analysis of US MarketScan claims data for patients with NVAF and diabetes, newly initiating therapy with rivaroxaban (n=10,017) or warfarin (n=11,665).
Patients with CKD Stage 5 or on haemodialysis were excluded.
Rivaroxaban was associated with lower risks of AKI and progression to Stage 5 CKD or haemodialysis versus warfarin in patients with NVAF and diabetes.
Sensitivity analysis using an intention-to-treat approach, excluding patients with AKI at baseline and limited to patients with >365 days of follow-up, yielded consistent results.
AKI: acute kidney injury; CI: confidence interval; CKD: chronic kidney disease; NVAF: nonvalvular atrial fibrillation; PY: patient years.
Adapted from Hernandez et al.29
Diabetes, Chronic Kidney Disease, and Cardiovascular Disease Risk
There is a strong correlation between diabetes and CV disease. Macrovascular complications, namely CAD, PAD, and stroke, are a consequence of the injurious effects of hyperglycaemia, along with microvascular complications including diabetic nephropathy, neuropathy, and retinopathy.30 Diabetes has been identified as a strong and consistent independent risk factor for stroke in patients with AF,31 and is also independently associated with an increased risk of AF.32 Furthermore, diabetes is a significant CV risk factor in patients with PAD or CAD.33
Evidence from a large population-level cohort study (N=1,268,029) showed that patients with both diabetes and renal impairment have an even greater CV risk than those with either diabetes or renal impairment alone. The study found that patients with a previous myocardial infarction represent a very high-risk group; patients with both diabetes and CKD were shown to be at similar or even higher risk of CV events and all-cause mortality.34
Dr Patel noted that rivaroxaban has been shown to be effective in patients with NVAF and diabetes in both randomised control trials35 and real-world36 studies, with consistent results. The ROCKET AF Phase III trial, which compared the effectiveness and safety of rivaroxaban and warfarin, enrolled 39.9% of patients with both NVAF and diabetes (n=5,695). A subanalysis of this cohort showed the efficacy and safety of rivaroxaban compared to warfarin was similar in patients with and without diabetes, supporting use of rivaroxaban as an alternative to warfarin in patients with these coexisting conditions.35
Similarly, results from a USA administrative claims database analysis showed that the effectiveness and safety of rivaroxaban was at least as good as warfarin in patients with NVAF and diabetes (n=11,034) treated in routine clinical practice. Rivaroxaban was associated with nonsignificant reductions in stroke or SE compared to warfarin (0.87 versus 1.35 events per 100 patient-years), with no differences in major bleeding. Reduced-dose rivaroxaban (15 mg od) was associated with a significantly decreased hazard of stroke or SE and IS, without an increase in major bleeding risk.36
Dr Patel shared results from a retrospective claims database analysis of patients with NVAF and diabetes investigating the effectiveness and safety of rivaroxaban and warfarin for prevention of major adverse CV events or major adverse limb events. Rivaroxaban use was associated with a lower risk of both major adverse CV events and major adverse limb events, with no difference in major bleeding.37
Returning to the patient case outlined previously, Dr Patel reminded delegates that it is important to determine what the patient is most concerned about, noting that most patients worry about being a burden to their family. He suggested that if there is concern about mobility, stroke reduction, and the kidneys, there could be an argument to proceed with anticoagulants for the patient. He added: “Although we’ve been talking about AF-related stroke prevention for 10 years there’s still so much to learn and progress we have to make to better optimally care for these multimorbid patients.”
Panel Discussion Highlights
The panel noted that CV risk management for patients recently diagnosed with T2DM includes glycaemic control, smoking cessation, blood pressure control, reduction in serum lipid with a statin, diet, exercise, and weight loss or maintenance, but does not include decisions about anticoagulation. Dr Rossing emphasised that the choice of an anticoagulant is an important consideration and should perhaps be part of this conversation since it can impact renal outcomes and thereby plays an important role in a patient’s progression down the line.