Meeting Summary
Advanced stage heart failure accounts for 1-10% of the overall heart failure population and the prevalence is increasing, in part because of improved treatment options, which have led to longer life expectancy in today’s patients compared to those of a generation ago. Recently, major improvements in technology and in the understanding of risk profiles have led to advancements in the use of mechanical circulatory support (MCS) devices, most notably the left ventricular assist devices (LVAD). The present article summarises key data, insights, and experiences recently presented by an expert panel at the virtual symposium ‘Surrounding Advanced Heart Failure’, held during the European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting 2020. The symposium focussed on how today’s LVAD therapies fit into the cardiological continuum, how to minimise the risk of adverse events, and how the impact of the coronavirus disease (COVID-19) pandemic might be mitigated by novel treatment approaches.
Introduction
Over the last half century, the morbidity and mortality of patients with congestive heart failure have been greatly improved by disease-modifying drugs and innovative device therapies; and yet, heart failure remains a progressive disease. Patients have progressed towards advanced-stage heart failure, a condition that currently accounts for 1-10% of the overall population of patients with heart failure. Improved treatment has contributed to an increased prevalence, as today’s patients live longer than those of the previous generation.1 Device therapies have long been an option for patients with advanced heart failure, but in the last decade there have been seismic shifts in the treatment landscape. Several clinical trials and registries have confirmed a large improvement in the risk/benefit profile of MCS devices, in particular LVAD. Recently, these advancements were counterbalanced by the advent of the COVID-19 pandemic, which has reduced access to care for high-risk groups, particularly end-stage patients awaiting elective surgery. The pandemic has generated new challenges for the multidisciplinary teams involved in the care of vulnerable patients.
Selecting Candidates for Ventricular Assist Device Therapy
In the early stages of heart failure, disease-modifying therapies and symptom management are important to slow progression and preserve quality of life. However, at the transition to advanced heart failure, oral pharmacotherapy starts to fail, the patients’ quality of life deteriorates markedly, and major treatment decisions are required. The options of heart transplant or MCS, either temporary (extracorporeal membrane oxygenation [ECMO]) or long-term (LVAD), all require careful evaluation.
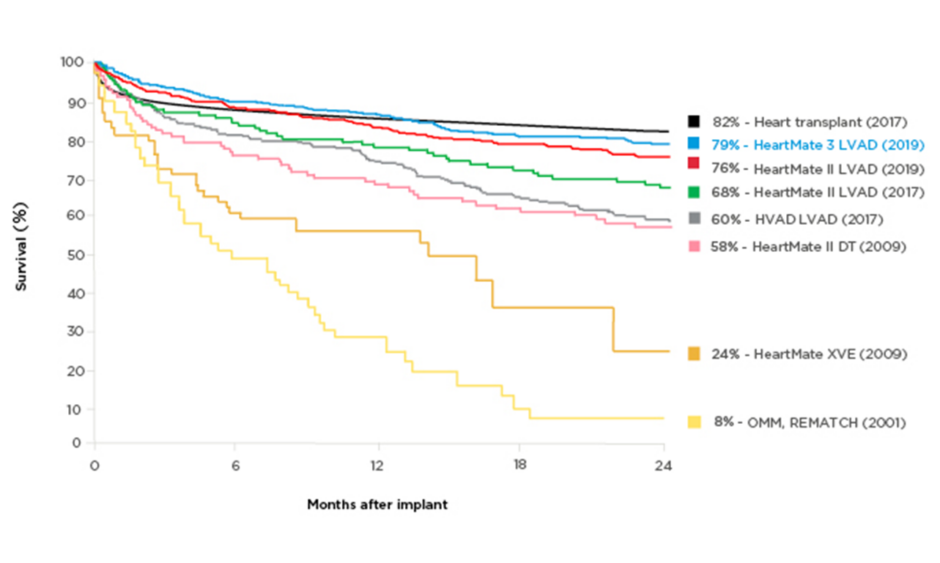
Technology improvements have made a profound difference to patients’ survival chances in the last decades. Two-year survival on an assist device was 23% in 2001 (8% on optimal medical management),2 whereas the latest generation of LVAD has a reported 2-year survival rate of 79%, rivalling the rate for heart transplants (82%) (Figure 1).2-6 Postmarket studies with LVAD have confirmed the greatly improved survival: 83% at 2 years in the ELEVATE registry of HeartMate 3⢠LVAD (Abbott, Abbott Park, Illinois, USA).7,8 This is an extremely longed-for development, as transplantation has always been a limited therapeutic option for patients with end-stage chronic heart failure. Despite the continuing advances, selection of appropriate candidates for LVAD therapy remains difficult and the decision requires a multidisciplinary approach. As with any interventional therapy, it is important to identify comorbidities that should be considered carefully, as well as the ‘sweet spot’, when patients are neither too ill nor too well to derive meaningful benefits from treatment. The optimal LVAD candidates are those expected to have poor outcomes without intervention and favourable outcomes with the intervention, and who are not contraindicated.9 Ideally, patients should be referred at an early disease stage to reduce the risk associated with the procedure.
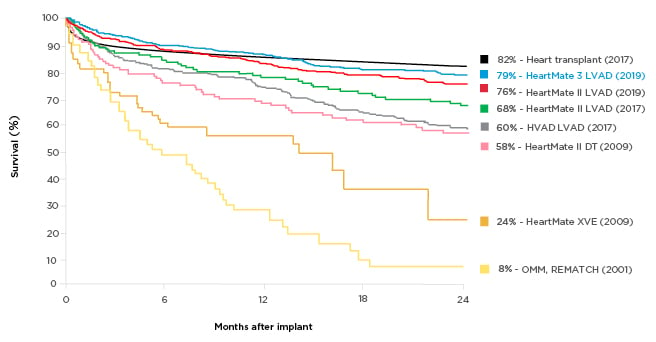
Figure 1: Survival with different generations of left ventricular assist devices compared with medical management.
A historical perspective of the survival of patients with end-stage heart failure who partook in randomised clinical trials. The yellow lines depict the survival associated with optimal medical therapy and pulsatile technology. Eight years after the completion of the REMATCH trial,2 pulsatile technology was replaced in favour of smaller continuous flow pumps, which translated into not only a dramatic improvement in survival as seen in the purple, green, and red curves, but also a drastic improvement in quality-of-life metrics. This new HeartMate (Abbott, Abbott Park, Illinois, USA) technology is associated with a 2-year survival of 83%, which now parallels that of heart transplantation.
DT: destination therapy; HVAD: HeartWare® [Medtronic, Dublin, Ireland] left ventricular assist device; LVAD: left ventricular assist device; OMM: optimal medical management.
Adapted from Rose et al.,2 Lund et al.,3 Mehra et al.,4 Rogers et al.,5 and Slaughter et al.6
The easiest decision for an LVAD is with patients identified as New York Heart Association (NYHA) Class IV who are haemodynamically stable but need low or intermediate doses of inotropes because of hypotension, worsening of symptoms, or progressive renal failure.10 For more severe, as well healthier, patients, the decision needs to account for the risk of adverse events with the therapy. Fortunately, these are becoming less frequent with newer device generations.
Improved Adverse Event Profile of Modern Left Ventricular Assist Devices
The associated risk starts with the implant procedure. Here, modern devices are increasingly designed for less invasive operations. One method relies on bilateral mini-thoracotomy in the fourth or fifth left intercostal space and the second right intercostal space. This grants access to the LV apex, as well as to the ascending aorta.11 The less invasive method preserves the pericardium and seemingly requires less intraoperative blood products.11 The approach has further been shown to be associated with reduced rates of postoperative right ventricular failure,12 one of the most common and serious complications of LVAD therapy.13
Recently, Rieband et al.11 showed that patients who had experienced less invasive LVAD implantation had better outcomes in subsequent heart transplants. In addition to the reduced need for blood products, there was less formation of antibodies against the donated heart than in patients operated on in the traditional way.11
Beyond the implant procedure, patients may be at risk of device malfunction, infection, bleeding, or stroke, with sometimes fatal consequences.13 This was particularly true for earlier LVAD devices and development efforts in recent years have focussed on reducing the risk of adverse events. Today, there is good evidence of lower rates of suspected pump thrombosis and stroke with the latest generation HeartMate 3. In the randomised MOMENTUM 3 trial5 and the international, real-world, all-comers ELEVATE registry,8 rates of suspected pump thrombosis and stroke were lower than in earlier LVAD generations (Table 1). The results were highly similar in both the randomised trial and in real-world use; rates of gastrointestinal bleeding in real-life use were less than one-half of those in the controlled trial.
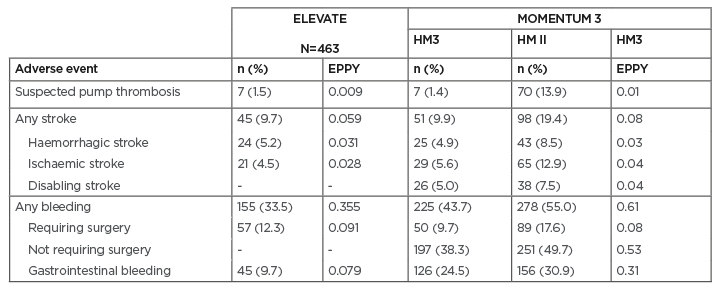
Table 1: Two-year adverse event rates in the ELEVATE registry and the MOMENTUM 3 trial.
EPPY: events per patient-year; HM3: HeartMate 3™ left ventricular assist device (Abbott, Abbott Park, Illinois, USA); HM II: HeartMate II™ left ventricular assist device (Abbott).
Adapted from Mehra et al.4 and Zimpfer at al.8
A comparison of adverse event rates with different types of devices was published in the latest annual report from the Society of Thoracic Surgeons (STS) Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs).7 In the analysis, patients who had received a centrifugal flow with a full magnetic levitation device (HeartMate 3) had the highest rates of freedom from first stroke, gastrointestinal bleeding, and major infection at 12 months, and the trend continued at 18 months.
Given the limited follow-up for the newest devices, comparisons beyond 1 year are limited and data from longer follow-up will be necessary to confirm the trends.
Netuka et al.14 have been investigating the possibility of decreasing anticoagulant use to reduce the risk of bleeding without increasing that of pump thrombosis or stroke. Results are available from an initial open-label trial in 15 patients who received a vitamin K antagonist at the target international normalised ratio 2.0-3.0 for 6 weeks after implant of a HeartMate 3 and who were then transitioned to a lower international normalised ratio target range of 1.5-1.9. After 6 months, 93% remained free from pump thrombosis, disabling stroke, or major bleeding.14 Larger studies are ongoing, including an investigation as to whether antiplatelet therapy can be withdrawn while maintaining vitamin K antagonist therapy.
Adverse events have not been eliminated and it is important to address them using a team approach; a dedicated team including cardiologists and referral centres should be involved. The establishment of Networks of Excellence is highly encouraged.
Use of Modern Left Ventricular Assist Devices
Originally considered only as a lifesaving therapy for patients ineligible for heart transplantation, LVAD are now indicated for bridge-to-transplant, bridge-to-recuperation, or as destination therapy. As noted above, patients in a severe condition may be too ill to benefit from LVAD. Patients requiring urgent transplant have significantly worse survival than elective transplants.15 However, it has been shown that temporary ECMO may improve the severity score of patients who are very ill, making them more suitable LVAD candidates. In a study that stabilised patients in severe conditions with ECMO before implanting an LVAD, mid-term survival after implant was comparable to that of less ill patients.16
In alignment with these findings, guidelines have recommended that unstable patients with cardiogenic shock should receive ECMO support before considering further therapies (bridge-to-decision).17 If the neurological function is favourable, the stabilised patient can be referred for long-term or bridge-to-therapy LVAD. However, for stabilisation before transplant in patients with advanced heart failure, ECMO may not be the best option. This has been shown by Crespo-Leiro et al.1 in critically ill, profoundly haemodynamically compromised patients with end-organ dysfunction in urgent need of transplantation. The best option was temporary bridging with LVAD, which was associated with significantly higher survival rates than bridging with ECMO or temporary biventricular assist devices.18 This corresponds to the recommendations in current heart failure guidelines.10
Left Ventricular Assist Devices in a COVID-19 World
The COVID-19 pandemic has upended healthcare systems worldwide, either as a result of the ‘lockdown’ measures or by directing resources towards infected patients. During lockdown, care delivery systems were reorganised in unprecedented ways, particularly in the cardiovascular community. There was a dramatic reduction in the availability of donor hearts: there were fewer car accidents, additional safety measures aimed at excluding potentially asymptomatic severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) carriers, and fewer non-COVID-19 patients admitted to intensive care units.19 As advanced heart failure is a condition with high morbidity and mortality, the situation raised urgent questions about how to treat these vulnerable patients during the crisis.
Given the severity of the situation, Dr Bories and Dr Akar considered the possibility of LVAD therapy, which is readily available and has a good survival rate at 1 year. Additionally, the operation is elective, meaning surgical and hospital capacity can be planned in advance. The team chose to implant LVAD in six patients with severe heart failure with left monoventricular dysfunction. The objective was to stabilise their condition and keep them safe at home, as well as to reduce the duration of hospitalisation and thus minimise their exposure to the virus. One of the patients had developed irreversible and refractory cardiogenic shock 1 month after SARS-CoV-2 infection; the others had negative virus tests (Table 2).
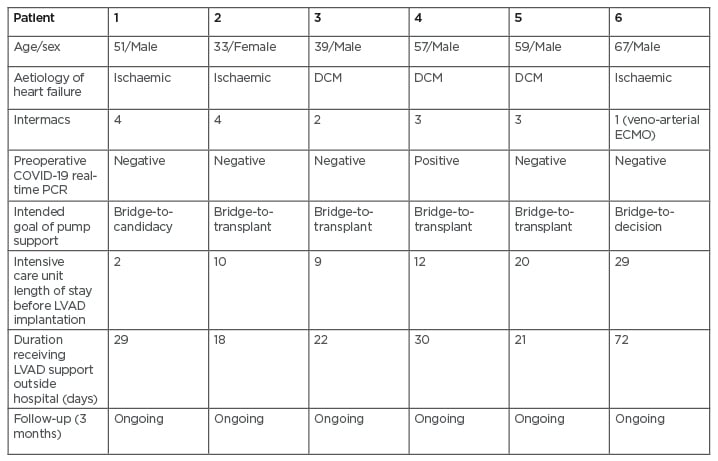
Table 2: Characteristics and outcomes of patients receiving left ventricular assist devices during the national lockdown in France.
COVID-19: coronavirus disease; DCM: dilated cardiomyopathy; ECMO: extracorporeal membrane oxygenation; Intermacs: Interagency Registry for Mechanically Assisted Circulatory Support; LVAD: left ventricular assist device.
All six HeartMate 3 implantations were successful, with five of six patients discharged within 30 days of the procedure; survival at the time of writing was 100%. The postoperative status of the patient infected with SARS-CoV-2 was uncomplicated. Importantly, none of the other five patients contracted the virus during or after implantation. This may have been because of the strict social distancing, limited family visits, and strict precautions taken by the caregivers. Follow-up has been continued through telemedicine methods and physical visitation.
The experience showed that, in spite of the COVID-19 pandemic, cardiologists still have the tools to provide appropriate therapies to patients. LVAD may be the treatment of choice for advanced heart failure because of the lack of donor hearts.
Conclusions
The management of advanced heart failure is evolving at a rapid pace. Pulling together the new knowledge and wide experience from recent years will require a multidisciplinary effort. Many questions on LVAD therapy remain; high on the wish list is to minimise the risks associated with the implant procedure and subsequent infection. By learning how to avoid right ventricular failure, how best to manage bleeding versus thrombosis risk, and what blood pressure levels to target, current LVAD devices will be able to provide maximal benefits in the most suitable patients. If future technological advancements are of the same magnitude as those of the last 20 years, the outlook for patients with advanced heart failure will be transformed.