Meeting Summary
Prof Nordestgaard said that genetic studies have shown that elevated triglyceride-rich lipoproteins can lead to atherosclerosis and inflammation, which can lead to myocardial infarction (MI). Genetic studies have also shown that lower triglyceride levels are associated with lower cardiovascular risk. Dr Bhatt then said that although low-dose omega-3 fatty acids (1 g/day) are ineffective for preventing heart disease, higher doses (1.8 g/day) have been shown to reduce coronary plaque and the risk of coronary events. He then described the recently published REDUCE-IT trial, which randomised ~8,000 statin-treated patients with elevated triglycerides (1.52–5.63 mmol/L) to icosapent ethyl 4 g/day or placebo. Those randomised to icosapent ethyl had significant reductions in triglyceride levels and cardiovascular events. American and European guidelines have now recognised that omega-3 fatty acids 4 g/day can be beneficial for the management of hypertriglyceridaemia and that icosapent ethyl, in particular, lowers the rate of cardiovascular outcomes. Dr Gitt presented data showing how many patients from DYSIS, a cross-sectional, observational study of lipid goal achievement among statin-treated patients, could benefit from icosapent ethyl. Among >60,000 patients in DYSIS, 72% were at very high cardiovascular risk, and 48% of these had triglycerides >1.52 mmol/L and could therefore potentially benefit from icosapent ethyl. Finally, Dr Konishi presented imaging data showing that eicosapentaenoic acid (EPA), of which icosapent ethyl is a purified ester, is associated with decreased plaque instability. This could help to explain how icosapent ethyl reduces cardiovascular risk.
Triglycerides, Lesson from Genetics
Professor Børge G. Nordestgaard
Based on genetic evidence, it is now known that triglyceride-rich lipoproteins lead to atherosclerosis and local inflammation, which can result in MI due to plaque rupture. Genetic studies have also shown that higher triglyceride levels are associated with a higher risk of MI and lower levels are associated with lower cardiovascular risk. Elevated low-density lipoprotein cholesterol (LDL-C), remnant cholesterol, and triglyceride levels can all increase the risk of cardiovascular disease. Chylomicrons, lipoprotein particles that consist largely of triglycerides, can also increase the risk of pancreatitis. In clinical practice, LDL-C, remnant cholesterol, and lipoprotein(a) are all important, but likely cause disease by different mechanisms. When triglycerides are degraded into toxic free fatty acids, they can cause inflammation, potentially leading to MI or acute pancreatitis. A second pathway by which triglycerides could lead to MI is via cholesterol, foam cells, and atherosclerosis.
In the Copenhagen General Population Study (N=84,177), there was a skewed distribution of nonfasting triglyceride levels.1 Most people (73.0%) had triglyceride levels of 0–2 mmol/L, while 27.0% had higher levels (2–10 mmol/L) that could be associated with an increased risk of cardiovascular disease. Only 0.1% had triglyceride levels >10 mmol/L, and such people are at increased risk of acute pancreatitis. In the combined Copenhagen General Population Study and the Copenhagen City Heart Study, higher nonfasting triglyceride levels were associated with higher plasma C-reactive protein (n=115,818), but there was no such correlation between LDL-C and C-reactive protein (n=115,377).2
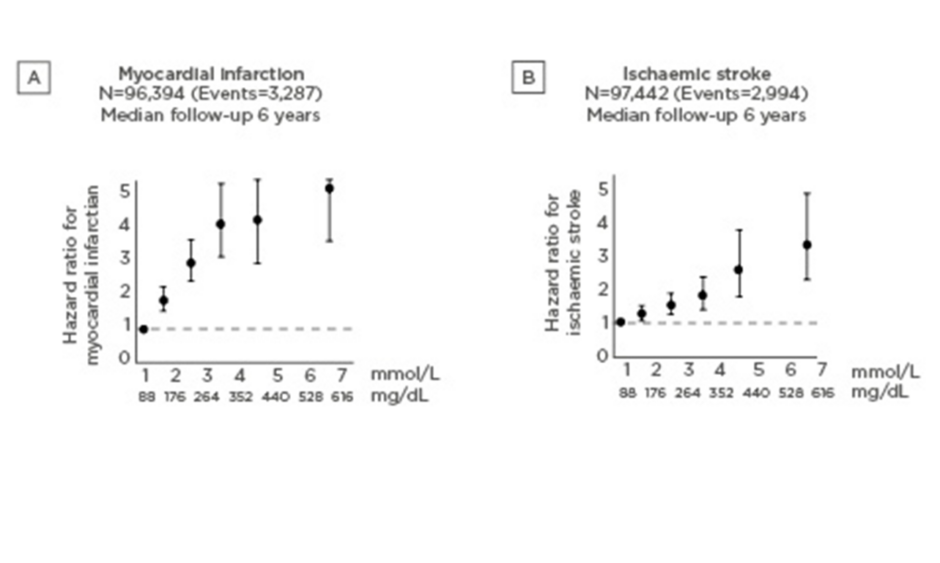
Among 116,550 people from the Copenhagen General Population Study, the risks of MI and acute pancreatitis increased significantly with increasing nonfasting triglyceride levels.3 Compared to people with triglycerides <1.00 mmol/L, those with triglycerides ≥5.00 mmol/L had a 3.4-fold higher risk of MI and an 8.7-fold higher risk of acute pancreatitis.3 Similarly, in the Copenhagen City Heart Study and the Copenhagen General Population Study, increasing levels of nonfasting triglycerides were associated with an increased risk of MI and stroke.1,4 These risks increased with increasing nonfasting triglyceride levels to ~5-fold for MI (Figure 1A) and ~3-fold for stroke (Figure 1B) for the highest versus lowest triglyceride levels.
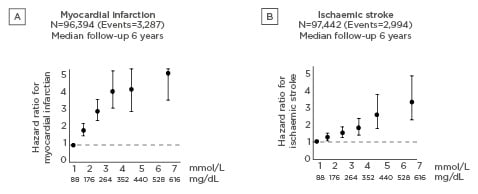
Figure 1: Increasing risk of myocardial infarction (A) and ischaemic stroke (B) with increasing nonfasting triglyceride levels.1,4
People with triglyceride levels <10 mmol/L tend to have smaller lipid particles and more LDL-C; those with higher levels (10–30 mmol/L) have more very low-density lipoprotein (VLDL)/remnants; and those with very high levels (>30 mmol/L) have more chylomicrons (unpublished data). While the risk of acute pancreatitis increases with increasing triglyceride levels, the risk of MI peaks at around 30 mmol/L and then decreases at higher triglyceride levels.
In the Copenhagen General Population Study (N=106,216), remnant cholesterol (calculated as total cholesterol – LDL-C – high-density lipoprotein cholesterol [HDL-C]) increased with increasing BMI, from 0.43 mmol/L among the lowest BMI decile to 0.88 mmol/L in the highest BMI decile (ptrend<0.001).5
Using Mendelian randomisation, the impact of genetic variants on triglyceride, LDL-C, and HCL-C levels can be studied.6 Provided enough people are studied, those with and without various genetic variants should be matched. In an old analysis from the Copenhagen City Heart Study and the Copenhagen General Population Study (N=56,657), the risk of ischaemic heart disease was increased 1.1–1.6-fold among those with elevated LDL-C, remnant cholesterol:HDL-C, or remnant cholesterol.7 Genetically elevated levels of remnant cholesterol:HDL-C or remnant cholesterol, however, were associated with much higher risks (2.8–2.9-fold) than genetically elevated LDL-C levels (1.5-fold) (n=54,924), showing the importance of remnant cholesterol.7
Other genetic studies support these data, showing that triglyceride-rich remnants can cause cardiovascular disease, independently of LDL-C and HDL-C.8-14
For example, two studies have examined loss-of-function mutations in the APOC3 gene and risk of ischaemic vascular disease.8,9 Both showed that heterozygosity for loss-of-function mutations in APOC3 was associated with ~40% reductions in both nonfasting triglyceride levels and risk of ischaemic vascular disease.8,9 Another study showed 27% lower triglyceride levels for ANGPTL3 loss-of-function and, based on a meta-analysis with other studies, a 39% lower risk of coronary artery disease.14
When people consume an excess of high-fat foods, chylomicrons and VLDL particles are produced. Lipoprotein lipase converts these into chylomicron remnants and intermediate-density lipoprotein, causing atherosclerosis. Some proteins enhance the action of lipoprotein lipase (e.g., apolipoprotein V and GPIHBP1), while others inhibit it (e.g., apolipoprotein C3, ANGPTL3, and ANGTTL4). If these inhibitory proteins can themselves be inhibited, then lipoprotein lipase will degrade triglycerides faster and potentially enhance clearance of chylomicrons, VLDL, chylomicron remnants, and intermediate-density lipoprotein in the circulation, reducing atherosclerosis.
Prof Nordestgaard then introduced three new and ongoing trials for triglyceride-reducing therapy to reduce major atherosclerotic cardiovascular event risk after statin treatment. The REDUCE-IT trial, which will be discussed in detail by Dr Bhatt, randomised >8,000 statin-treated patients to icosapent ethyl or placebo and showed a reduction in major ischaemic events.15 The STRENGTH trial randomised ~13,000 patients to omega-3 carboxylic acids (Epanova®) and statin or corn oil and statin.16 The PROMINENT trial is currently recruiting ~10,000 participants, who will be randomised to the selective peroxisome proliferator alpha modulator (SPPARM-α), pemafibrate, or placebo.17
Prof Nordestgaard finished with the same slide that he started with, but expanded it to say that the link from triglyceride-rich lipoproteins to atherosclerosis with inflammation to MI due to plaque rupture could theoretically be attributable to various factors: coagulation, arrythmias, other inflammation, HDL-C, or small dense LDL-C; there is however no genetic evidence to support such pleiotropic effects. He reiterated that higher levels of triglyceride-rich lipoproteins increase the risk of MI, and reducing triglyceride-rich lipoprotein levels reduces the risk of MI, based on genetic data.
Does High-Dose Fish Oil Reduce Cardiovascular Events via Triglycerides?
Doctor Deepak L. Bhatt
In 2015, Prof Libby suggested that reducing triglycerides may be important for reducing cardiovascular risk.18 However, in December 2018, the European Medicines Agency (EMA) reported that omega-3 fatty acid mixtures of EPA and docosahexaenoic acid (DHA) (1 g/day) are not considered effective in preventing heart disease.19
In a naturally randomised trial, Ference et al.20 evaluated the risk of coronary heart disease among people with and without genetic variants that are associated with lower triglyceride levels (via the lipoprotein lipase pathway) and/or lower LDL-C (via upregulation of the LDL receptor). Both variants significantly reduced the risk of coronary heart disease, showing that triglycerides and LDL-C are both important. However, the implication was that for a similar reduction in coronary heart disease risk, triglyceride levels would need to be reduced ~5-fold more than LDL-C levels.
In a recent meta-analysis of 10 trials, low-medium-dose omega-3 fatty acid preparations (EPA 226–1,800 mg/day) had no significant effect on the primary cardiovascular endpoints.21 However, in the randomised Japanese JELIS study (N=18,645), EPA (1.8 g/day) plus statin significantly reduced the incidence of coronary events versus statin alone (with no placebo) among patients with hypercholesterolaemia (2.8% versus 3.5%; p=0.011).22 This agrees with mechanistic data from the newer randomised CHERRY study, in which medium-dose EPA (1.8 g/day) plus statin resulted in significantly more coronary plaque regression than statin alone (81% versus 61%; p=0.002) among 193 patients with coronary heart disease after percutaneous coronary intervention (PCI).23
In the REDUCE-IT study, 8,179 statin-treated patients (aged ≥45 years with established cardiovascular disease or ≥50 years with diabetes and ≥1 additional risk factor) with triglycerides 1.52–5.63 mmol/L and LDL-C 1.06–2.59 mmol/L were randomised to icosapent ethyl (4 g/day) or placebo, both with continuing statin therapy.15 The primary endpoint was a composite of cardiovascular death, nonfatal MI, nonfatal stroke, coronary revascularisation, or hospitalisation for unstable angina; the key secondary endpoint was a composite of cardiovascular death, nonfatal MI, or nonfatal stroke.15 The median age was 64 years, 71% were male, and 58% had Type 2 diabetes mellitus.15 The secondary prevention cohort accounted for 71% of the population, whereas the other 29% were in the primary prevention cohort.
In the REDUCE-IT study, icosapent ethyl resulted in a 19.7% reduction in triglycerides versus placebo (p<0.001).15 There were also significant reductions in various other biomarkers and a significant increase in EPA (359%; p<0.001). Icosapent ethyl also resulted in significant reductions in the primary composite endpoint (17.2% versus 22.0%; hazard ratio [HR]: 0.75; 95% confidence interval [CI]: 0.68–0.83; p<0.001; Figure 2) and the key secondary endpoint (11.2% versus 14.8%; HR: 0.74; 95% CI: 0.65–0.83; p<0.001).15
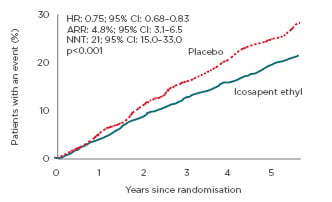
Figure 2: Reduced risk of the composite primary endpoint (cardiovascular death, myocardial infarction, stroke, coronary revascularisation, or unstable angina) with icosepent ethyl versus placebo in the REDUCE-IT trial.15
ARR: absolute risk reduction; CI: confidence interval; HR: hazard ratio; NNT: number needed to treat.
There were also significant reductions in various other composite and single endpoints. In subgroup analyses, icosapent ethyl had a significant effect on the primary and key secondary endpoints among those with baseline triglyceride levels < or ≥2.26 mmol/L.15 It also had a significant effect on the key secondary endpoint among those with baseline triglyceride levels < or ≥1.69 mmol/L.15 Similarly, Kaplan–Meier curves of the primary and key secondary endpoints were very similar for those with achieved triglyceride levels < or ≥1.69 mmol/L.15
Icosapent ethyl did not only significantly reduce first events (HR: 0.75; 95% CI: 0.68–0.83), but also significantly reduced second (HR: 0.68; 95% CI: 0.60–0.78), third (HR: 0.69; 95% CI: 0.59–0.82), and fourth or more (relative risk [RR]: 0.52; 95% CI: 0.38–0.70) events.24 Overall, there was a 30% reduction in total events in the icosapent ethyl group versus the placebo group (RR: 0.70; 95% CI: 0.62–0.78; p<0.0001).24 For every 1,000 patients treated with icosapent ethyl for 5 years, one could expect 76 fewer coronary revascularisations, 42 fewer MI, 16 fewer hospitalisations for unstable angina, 14 fewer strokes, and 12 fewer cardiovascular deaths (overall, 159 less events).24
In the REDUCE-IT trial, the original triglyceride inclusion criterion was 1.69–5.63 mmol/L at the screening visit which,25 with a 10% allowance, resulted in those with triglycerides ≥1.52 mmol/L at this visit being included.15 However, when the baseline triglyceride level was calculated as the mean of the screening and randomisation visit values (the latter of which did not have defined limits), baseline values actually ranged from 0.91 to 15.82 mmol/L.26 There were significant relative reductions in the primary composite endpoint with icosapent ethyl in all three baseline triglyceride tertiles (lowest: HR: 0.79; 95% CI: 0.66–0.94; p=0.0069; middle: HR: 0.80; 95% CI: 0.68–0.95; p=0.0121; highest: HR: 0.68; 95% CI: 0.57–0.80; p<0.0001), with no significant interaction by subgroup (pinteraction=0.33). Results for total events were similar (lowest: HR: 0.74; 95% CI: 0.61–0.90; p=0.0025; middle: HR: 0.77; 95% CI: 0.63–0.95; p=0.0120; highest: HR: 0.60; 95% CI: 0.50–0.873; p<0.0001; pinteraction=0.17).26 Comparing absolute (rather than relative) risk reductions, there was no significant interaction by subgroup for the first event (pinteraction=0.12), but a larger effect at higher baseline triglyceride levels for total events (pinteraction=0.03).26 Of note, among patients on placebo, the rate of total endpoint events increased from 75 to 87, to 107, per 1,000 patient years in the lowest to highest triglyceride tertiles, showing the detrimental impact of triglyceride level on cardiovascular risk.
A recent publication has reviewed eight key triglyceride-lowering trials (three fibrates, three omega-3 fatty acids, and two niacin).27 Most of the studies reported significant reductions in triglyceride levels, but only one fibrate and three omega-3 fatty acid studies (including REDUCE-IT) reported significant effects on cardiovascular outcomes.
The biological effects of EPA on plaque progression include those on:
Endothelial dysfunction/oxidative stress (e.g., increased endothelial function and nitric oxide bioavailability; decreased oxidised LDL, macrophages, and foam cells).
Inflammation/plaque growth (e.g., increased EPA/arachidonic acid [AA] ratio; decreased IL-6).
Unstable plaque (e.g., increased fibrous cap thickness and plaque stability; decreased plaque volume and arterial stiffness).28
It has also been associated with modest placebo-corrected reductions in blood pressure (systolic blood pressure: 1.3 mmHg; diastolic blood pressure: 0.5 mmHg).29 Dr Bhatt noted that EPA and DHA have different effects on cellular membranes.30 EPA tends to be associated with the hydrocarbon core, resulting in an ordered membrane, while DHA interacts with the headgroup region, resulting in a less ordered structure.30
He then went on to outline some ongoing studies. In the EVAPORATE study, ~60 statin-treated patients with coronary atherosclerosis and elevated triglycerides were randomised to icosapent ethyl 4 g/day or placebo, with continued statin therapy.31 Multidetector CT angiography will be used at 9 and 18 months to assess changes in plaque volume and various other secondary endpoints. In the STRENGTH trial, ~13,000 statin-treated adults with elevated triglycerides and low HDL-C levels were randomised to omega-3 carboxylic acids 4 g/day or placebo.16 The primary endpoint will be the time to first major cardiovascular event. In the PROMINENT study, ~10,000 statin-treated patients with Type 2 diabetes mellitus, elevated triglycerides, and low HDL-C are being randomised to pemafibrate or placebo.17 The primary endpoint will be the time to first major cardiovascular event.
The American Heart Association (AHA) has said that the prescription omega-3 fatty acid icosapent ethyl at 4g/d has been shown to reduce atherosclerotic cardiovascular disease risk among patients with elevated triglycerides, based on the REDUCE-IT trial.32 European guidelines recommend considering icosapent ethyl 4 g/day plus statin for those with triglycerides 1.50–5.60 mmol/L despite statin treatment.33 Similarly, a March 2019 update from the American Diabetes Association (ADA) recommends that icosapent ethyl (but not other omega-3 fatty acid products) should be considered for patients with atherosclerotic cardiovascular disease or other cardiac risk factors who have triglycerides 1.52–5.63 mmol/L despite taking a statin.34
Prevalence of Hypertriglyceridaemia in Statin Treated Very High-Risk Patients Who Might Benefit from Treatment with Icosapent Ethyl for Secondary Prevention in Clinical Practice – Results of DYSIS35
Doctor Anselm K. Gitt
To ascertain how many patients in clinical practice might benefit from icosapent ethyl, Dr Gitt and colleagues looked at statin-treated patients in the cross-sectional, observational DYSIS study, which examined lipid goal attainment in Canada, Europe, Middle East countries, and China.
Data were collected in physicians’ offices and hospital outpatient wards during 2008–2012.
DYSIS included 61,805 consecutive patients on statin treatment. Of these, 44,593 (72.2%) were at very high cardiovascular risk (defined as per 2011 European Society of Cardiology [ESC]/European Atherosclerosis Society [EAS] guidelines),36 including patients with coronary heart disease, diabetes, chronic kidney disease, or peripheral atherosclerotic disease.
Among these very high cardiovascular risk patients, 21,312 (47.8%) had elevated triglyceride levels (>1.52 mmol/L). Patients with triglyceride levels >1.52 mmol/L were significantly more likely to be female (42.2% versus 38.6%), obese (31.0% versus 20.7%), have sedentary lifestyles (43.0% versus 37.6%), hypertensive (79.0% versus 74.9%), and diabetic (56.3% versus 45.3%) than those with triglycerides ≤1.52 mmol/L (all p<0.0001). They also had significantly higher total (4.78 versus 4.11 mmol/L) and LDL-C (2.64 versus 2.30 mmol/L) levels (both p<0.0001); and were less likely to be at an LDL-C goal of <1.81 mmol/L (19.9% versus 28.8%).
Overall, nearly half of the very high cardiovascular risk patients in DYSIS, and over a third of the total DYSIS population, treated with statins for secondary prevention had elevated triglyceride levels and may therefore benefit from additional treatment with icosapent ethyl (Figure 3). As >80% of these very high-risk patients with triglycerides >1.52 mmol/L did not reach LDL-C targets, these patients could particularly benefit from icosapent ethyl. This could result in further reductions in major ischaemic events, such as cardiovascular death.
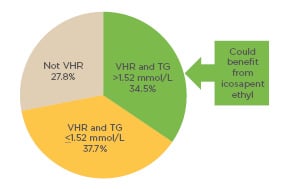
Figure 3: Pie chart showing the proportion of patients in the observational DYSIS study (which included 61,805 consecutive patients on statin treatment) who could potentially benefit from icosapent ethyl, based on their ESC/EAS-defined cardiovascular risk36 and their triglyceride levels.
EAS: European Atherosclerosis Society; ESC: European Society of Cardiology; TG: triglycerides; VHR: very high risk.
Eicosapentaenoic Acid Therapy is Associated with Decreased Coronary Plaque Instability Assessed Using Optical Frequency Domain Imaging37
Doctor Takao Konishi
Dr Konishi discussed a retrospective study that used optical frequency domain imaging (OFDI) to assess the relationship between EPA therapy and coronary plaque instability.37 They included 121 consecutive patients who underwent PCI during 2015–2018. Of these, 12 patients had received EPA and these patients were propensity score matched (1:4) to 48 of 109 who had not received EPA. The morphological characteristics of the plaque were analysed using OFDI.
Baseline characteristics (age, sex, BMI, diabetes, hypertension, chronic kidney disease, previous PCI or coronary artery bypass grafting, previous MI, prior statin use, acute coronary syndrome, and HbA1c) were balanced in the two groups.37 Triglyceride, LDL-C, and HDL-C levels were also well matched. Those who had taken EPA had a higher mean EPA/AA ratio (1.63±0.46 versus 0.48±0.21; p<0.001).
In terms of OFDI characteristics, patients who had received EPA had a significantly lower mean lipid index (818±806 versus 1,574±891; p=0.01), lipid length (3.8±2.8 versus 6.2±2.6 mm; p=0.007), maximum lipid arc (161±106 versus 236±84 degrees; p=0.011), and macrophage grade (13.5±5.9 versus 19.3±7.4; p=0.019), but a higher minimum fibrous cap thickness (109.2±55.7 versus 81.6±36.4 μm; p=0.4) than those who had not.37 Multiple logistic regression analyses showed that prior EPA use was independently associated with lower lipid index (p=0.043) and macrophage grade (p=0.024).
Overall, this analysis suggests that EPA therapy is associated with decreased plaque instability in patients with coronary artery disease undergoing PCI. Dr Konishi therefore suggested that patients with coronary artery disease who are at high risk of cardiovascular events should receive EPA to stabilise their coronary atherosclerotic plaques.
Dr Konishi also highlighted various previous studies that have examined the effects of EPA. Ferguson et al.38 reported that EPA+DHA attenuated the inflammatory activation of in vitro human adipocytes. Niki et al.39 randomised 95 patients on strong statin therapy to EPA or control, and found significant reductions in lipid volume and significant increases in fibrous volume in the EPA group, but not in the control group. They also found significant reductions in inflammatory cytokines in the EPA, but not in the control group.39 Wu et al.40 reported that EPA+DHA decreased apoptosis in one healthy subject. Lastly, Zampelas41 reported that EPA use is associated with increased stability and decreased inflammation. Overall, these results suggest that EPA could reduce lipid core size by reducing inflammation.
Dr Konishi also discussed studies in which omega-3 fatty acids downregulate the expression of inflammation-related genes through inhibition of NF-kB signalling by blocking Iκ-β phosphorylation, through GPR 120, or the nuclear receptor PPARα/γ.42-45 Lastly, Kanai et al.46 reported that EPA can inhibit the ability of macrophages to secrete matrix metalloproteinases (MMP) and monocyte chemotactic protein 1 (MCP-1), and Matsumoto et al.47 reported that EPA can attenuate upregulation of vascular cell adhesion protein 1 (VCAM-1), intercellular adhesion protein 1 (ICAM-1), and MCP-1, and the expression of MMP-2 and MMP-9 in macrophage-like cells.