Meeting Summary
This case-based satellite symposium chaired by Prof Konstantinides addressed important and topical aspects of the management of acute pulmonary embolism (PE) with a focus on effective management in a real-world setting. The objectives of the symposium were to provide information and expert guidance on the effective management of a patient with PE from diagnosis and assessment of severity, through to the practical use of non-vitamin K antagonist non-oral anticoagulants (NOACs) and the management of challenging cases found in routine clinical practice. Following Prof Konstantinides’ introduction, Dr Hughes presented a low-risk PE case and discussed assessment of the severity of PE, the optimisation of hospital care, and the importance of patient discharge protocols and clear integrated management pathways. Dr Jiménez went on to illustrate the use of risk assessment and non-vitamin K antagonist (VKA) therapies through consideration of an intermediate high risk PE case with comorbidities. Finally, Dr Eikelboom presented an unprovoked PE case and discussed the key question of ‘how long is long enough’, emphasising the importance of adequate anticoagulation, both acutely and in prevention of recurrence, and the potential benefits of NOACs. In a final Question and Answer Hub session, the audience were able to participate in a lively case-based discussion.
Opening and Introduction
Professor Stavros V. Konstantinides
PE is the third most frequent acute cardiovascular syndrome and is associated with significant mortality, so all patients should receive anticoagulant treatment irrespective of severity.1 Initially the NOACs were regarded as alternatives to standard of care,1 but more recently the American College of Chest Physicians (CHEST)2 has recommended them over the classical regimen of low molecular weight heparin (LMWH) combined with VKAs because of their equivalent efficacy and better safety profile, at least in terms of major bleeding. NOACs also allow for the possibility of earlier discharge and subsequent home treatment of selected patients with low-risk PE. This is currently being investigated in studies like the HoT-PE trial,3 the primary objective of which is to determine whether early discharge and subsequent out-of-hospital management of patients with rivaroxaban is feasible, effective, and clinically appropriate in those fulfilling specific clinical, imaging, and social criteria of low-risk. Key clinical questions remain on: i) the best selection criteria for patients who can be treated in the outpatient setting, ii) how to decide the duration of treatment, and iii) how we should manage challenging patients such as those with concomitant deep vein thrombosis (DVT) and PE, or those at higher risk because of comorbidities.
Case 1: Assessing Pulmonary Embolism Severity and Optimising Hospital Care
Doctor Rodney Hughes
Patients with acute PE vary tremendously from the very low risk, who tend to be younger and fitter, through to a high-risk population for whom immediate reperfusion may be required. There is also an at-risk group of patients with the potential to deteriorate and have a further event within a short period, particularly if the anticoagulation is incomplete initially. Rapid risk stratification is therefore critically important to inform and optimise patient care.
The Pulmonary Embolism Severity Index (PESI) is a simple and well-validated4 risk stratification tool enshrined in the European Society of Cardiology (ESC) guidelines,1 which can help determine the potential mortality and outcome of patients with newly diagnosed PE. Used either as the simplified version (sPESI) or the full version and combined with an assessment of how the patient is coping, it builds a risk score classifying patients as high risk, intermediate high risk, intermediate low risk, or low risk. Its utility is exemplified by the case of a 49-year-old woman with no significant background risk factors for venous thromboembolism (VTE) who presented with low-grade tachycardia and slightly delayed onset of breathlessness. Blood pressure and oxygen saturations were normal but there was a saddle PE upon radiology. However, this patient’s troponin was negative so, far from needing thrombolysis, her low PESI score indicated she was at a low risk of a significant adverse outcome.
The choice of anticoagulant is important and there is a plethora of data available on the new agents. Rivaroxaban has the only PE-specific study to date, EINSTEIN PE.5 In this event-driven trial comparing rivaroxaban to the standard of care of enoxaparin/VKA, rivaroxaban met the criteria for non-inferiority in terms of the primary outcome of symptomatic recurrent VTE, but with a significantly better safety profile based on major bleeds. Pre-randomisation use of heparin did not significantly affect outcome.6 Furthermore, a retrospective post-hoc analysis7 indicated that the study included significant numbers of higher-risk patients and whilst no statistical comparison could be made, the data suggest that rivaroxaban may be equally effective for both low and high-risk patients according to sPESI.
But do results in clinical practice mirror those seen in clinical trials? XALIA,8-9 is a large, real-world, Phase IV study comparing the safety of rivaroxaban with standard of care for the treatment of acute DVT in routine clinical practice. Although the age range of patients in XALIA was lower than average (55–60 years), the patients were otherwise representative of those seen in clinical practice. Results were consistent with those seen in the rivaroxaban Phase III programme, with an almost identical reduction in hospital length of stay for DVT and PE patients.8-12 Figure 1 presents a comparison of the EINSTEIN PE and XALIA trials.
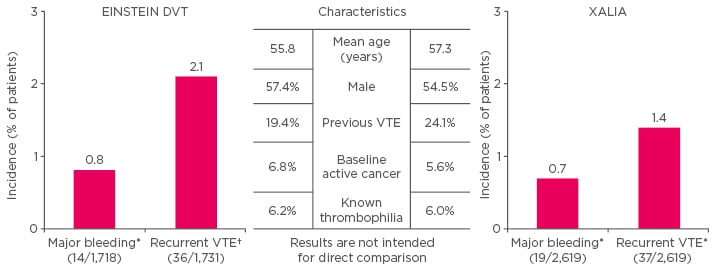
Figure 1: Comparison of the XALIA trial8-9 versus EINSTEIN DVT.10
*Safety population (patients taking ≥1 dose of study drug) †intention-to-treat analysis.
VTE: venous thromboembolism.
Adapted from The EINSTEIN Investigators 2010,10 Turpie et al.,8 and Ageno et al. 2016.9
Based on the weight of evidence for NOACs, the CHEST guidelines2 suggest that NOACs are preferred long-term anticoagulants over VKA for non-cancer associated VTE. Furthermore, use of these agents facilitates early discharge, either direct from the emergency department or after a couple of days’ inpatient care. Indeed, the ESC guidelines1 advise that patients with a low risk of an early adverse outcome should be considered for early discharge and continuation of treatment at home if proper outpatient care and anticoagulant treatment can be provided.
Rapid patient assessment and minimisation of in-hospital care starts in the emergency department with timely diagnostic procedures and PESI-scoring, followed by a decision about whether the patient can be discharged rapidly. Successful implementation of that early discharge requires establishment of integrated VTE management pathways with robust associated patient education, discharge, and follow-up processes and communications. These, combined with a holistic approach to the management of the patient, can ensure seamless and effective management of the patient from acute care right through to long-term management in primary care.
Case 2: Managing Challenging Patients with Venous Thromboembolism
Doctor David Jiménez
Acute mortality for normotensive patients with acute symptomatic PE varies widely, so risk stratification is critical to identifying both the very low-risk patient who might benefit from early discharge or home therapy, and the very high-risk patient who might deteriorate soon after diagnosis, and so requires close monitoring or urgent recanalisation procedures. The risk stratification algorithm from the ESC1 is very useful and may be refined in the near future to address questions regarding:
- Choice of scoring system for normotensive patients with PE, e.g. PESI, sPESI, or Hestia criteria
- In which patient subgroups should we combine clinical prognostic scores such as sPESI cardiac biomarkers and imaging?
- Definition of early discharge and home treatment for patients with acute symptomatic PE
- Better ways of differentiating between patients with intermediate high and intermediate low risk PE
Consider the case of a 76-year-old male admitted to the emergency department 2 days after a long haul flight with pleuritic chest pain and shortness of breath. Upon admission, his heart rate was 116 beats per minute, systolic blood pressure 99 mmHg, and oxygen saturation 93%. He had moderate renal impairment (creatinine clearance of 38 mL/min) and a high D-dimer of 3,122 ng/mL. Computed tomography (CT) scanning revealed a saddle pulmonary embolus. The patient was high-risk based on his sPESI and he had a positive troponin I (0.3 ng/mL) and right ventricular dysfunction on transthoracic echocardiography. Clinically, the question then was whether to thrombolyse.
The 2014 PEITHO trial13 is informative in this regard. Patients with intermediate-risk PE, defined as the presence of right ventricular dysfunction and myocardial injury, were randomised to either tenecteplase or placebo as well as anticoagulant therapy. Whilst the primary outcome of thrombolysis was positive with a significant reduction in all-cause mortality or haemodynamic collapse during the first 7 days after randomisation, there was also a significant increase in bleeding-related death.12 The ESC1 and recent CHEST2 guidelines therefore suggest not to thrombolyse normotensive patients with an acute symptomatic PE unless they deteriorate soon after diagnosis.
Based on the PEITHO13 trial experience, the therapeutic options for this patient were traditional parenteral agents with bridging and followed with VKA, rivaroxaban, apixaban, or 1 week’s LMWH followed by dabigatran or edoxaban.14 Whilst some clinicians are reluctant to use direct oral anticoagulation for acute symptomatic PE, there are good data supporting their efficacy, as exemplified by the Van Es et al.15 meta-analysis showing non-inferiority as compared to standard therapy with VKAs.
Not only has non-inferiority to VKA been reproducibly demonstrated for NOACs, there is also good evidence of actual clot regression.16 In the EINSTEIN PE trial, after 21 days of treatment with rivaroxaban 88% of patients achieved complete or partial clot resolution, and there was no worsening of pulmonary vascular obstruction in the remaining 12% of patients.16
The elderly are a particular safety concern in the use of anticoagulation in clinical practice, but a sub-analysis of the EINSTEIN trials is reassuring, with major bleeding rates on rivaroxaban reduced by 73% compared with warfarin in patients aged >75 years with low body weight or creatinine clearance <50 mL/min (Figure 2).17 Although patients with symptomatic VTE and renal impairment are at increased risk of recurrent VTE,18 pooled analysis of the EINSTEIN DVT9 and PE5 trials indicates that the risk of major bleeding is not increased in rivaroxaban-treated patients.18
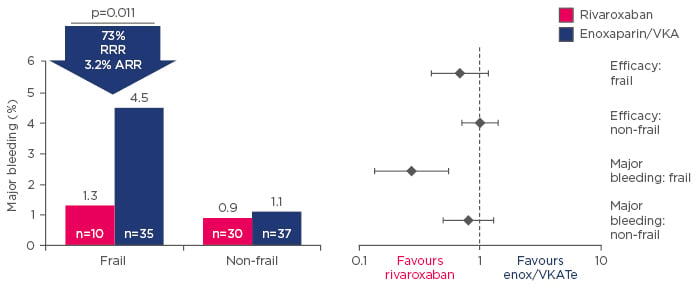
Figure 2: Sub-analysis of the EINSTEIN trials showing major bleeding events for rivaroxaban or enoxaparin/vitamin K antagonist-treated frail patients.17
ARR: absolute risk reduction; RRR: relative risk reduction; VKA: vitamin K antagonist; enox: enoxaparin.
Based on the evidence, the final decision for our patient was not to thrombolyse but to follow the CHEST guidelines2 and use weight-adjusted LMWH with monitoring. If there was no deterioration in 48–72 hours we would switch to NOACs and if there was deterioration, we would proceed to thrombolysis and to switch after 48 or 72 hours following stabilisation. Based on current guidelines,2 the vast majority of patients with acute symptomatic PE should be treated with NOACs and low-risk patients might benefit from early discharge or even from outpatient therapy. For those patients with high-risk PE or deteriorating patients with intermediate-risk PE, parenteral anticoagulant agents such as LMWH or unfractionated heparin may be more suitable in the acute setting, with initiation of NOACs after stabilisation.2
Case 3: How Long is Long Enough?
Doctor John Eikelboom
In the last 20 years there have been significant strides in the acute and long-term treatment of VTE, but one of the most contended issues for clinicians remains that of the optimal duration of anticoagulation. Over the years this has been driven by the inconvenience and high risk of bleeding associated with warfarin. Today, the advent of the NOACs has substantially overcome this and treatment has evolved towards a single-drug approach using rivaroxaban or apixaban commenced at outset and continued long-term.19
The 2016 CHEST2 guidelines are clear that treatment should be stopped at 3–6 months if the bleeding risk is high in a patient with an unprovoked event, but extended indefinitely if the bleeding risk is low or moderate. Where there is a transient risk factor, patients generally only require treatment for 3 months, but those with progressive or persistent risk factors should receive extended treatment.2 The CHEST guidelines2 now recommend NOACs over VKAs for the initial treatment of DVT or PE, and initial therapy choice can be extended where required, the only exception to this being cancer-associated thrombosis where LMWH remains the treatment of choice for extended/long-term treatment. A typical case of a 63-year-old male with unprovoked and fairly severe bilateral PE, elevated troponin, and evidence of right heart strain on imaging is illustrative of patients in whom the acute event has been treated after 3–6 months, but for whom there is a high risk of recurrence warranting extended treatment.
Decision-making based on estimated risk of recurrent VTE has been both enhanced and complicated by consideration of factors such as male sex and presence of positive D-dimer, but Kearon and Akl’s 2014 paper20 elegantly integrates that information with clinical information on risk of recurrence. Clinical risk factor status is the primary determinant of decision-making on treatment duration and our 63-year-old male has no provoking risk factor, therefore having a high recurrence risk in the first year of stopping of 10%, rising to 30% over 5 years and approximately 40% at 10 years.21
Historically, warfarin has been stopped after 3–6 months, but in the PADIS-PE trial,22 where extended treatment with warfarin to 18 months was compared with a placebo, event rates in the treated group increased gradually in the 24 months after stopping, reaching those of the placebo group by the end of the trial. This underlines the importance of ongoing long-term treatment in patients at risk of recurrence; however, bleeding risk, the need for injections, and the routine anticoagulation monitoring remain significant barriers to long-term anticoagulation with the older agents. Head-to-head trials confirm that NOACs such as rivaroxaban, apixaban, dabigatran, and edoxaban have similar efficacy to warfarin but a better safety profile, making them attractive for long-term treatment. Long-term treatment continues to be an important area of research and we particularly await the results of the EINSTEIN-CHOICE study,23 which compares the standard 20 mg dose of rivaroxaban with 10 mg of rivaroxaban and with 100 mg of aspirin over a 12-month period in 2,850 patients with symptomatic VTE and/or PE. The primary endpoints are symptomatic recurrent VTE and major bleeding. The trial is expected to be completed towards the end of 2016.23
Question and Answer Session
Q: If Dr Hughes’ patient (Case 1) had been thrombolysed, when would he have started the oral drug?
Dr Hughes’ practice is to anticoagulate with LMWH for 48–72 hours and then consider transitioning to a NOAC if the patient is stable.
Q: Would Dr Jiménez consider low-dose thrombolysis in his patient (Case 2)?
The panel agreed that although theoretically attractive, there are no good quality data to support this. They indicated that they would thrombolyse with full systemic doses.
Q: If an intermediate-risk patient on a NOAC starts to deteriorate in the first 48 hours, suggesting the need for thrombolysis, do you try to reverse the NOAC pre-thrombolysis?
Drawing an analogy to the myocardial situation, the panel agreed that in the case of a PE they would treat this as an emergency and simply treat with lysis, without attempting to reverse the NOAC.
Hub Question and Answer Session
After the symposium, delegates had the opportunity to explore additional case studies in a Question and Answer Hub session.
Case A
Dr Jiménez’ case concerned a 48-year-old woman who presented with ischaemic stroke after saphenectomy. Lower limb ultrasound proved negative for DVT but a multi-detector CT scan confirmed acute symptomatic PE, which was managed acutely with LMWH followed by VKA. However, saphenectomy is a transient risk factor so for how long should the patient be treated? Subsequent echocardiography revealed a foramen ovale, putting the patient at high risk of ischaemic stroke, so indefinite anticoagulation was the best course of action. Despite the potential risks, this patient underwent closure of the foramen ovale after 3 months and anticoagulant therapy could then be stopped. Dr Jiménez emphasised the importance both of reviewing bleeding occurrence in patients with unprovoked PE who continue treatment and event recurrences in those whose anticoagulation therapy is stopped.
Q: When would you consider anticoagulation in a patient with ischaemic stroke if you have a confirmed coexistent VTE?
Dr Jiménez responded that in the absence of good control data, his clinical practice is to start with full dose LMWH for 8 hours after the diagnosis of an ischaemic stroke and then switch to a direct oral anticoagulant 1 week after diagnosis of ischaemic stroke.
Q: Do you use scanning and imaging to follow-up these patients?
Dr Jiménez responded that most patients recover in terms of clinical signs and clot regression is assumed. He rarely rescans these patients unless they deteriorate and have a suspected recurrent VTE.
Case B
Dr Hughes presented the case of a 38-year-old woman who was usually fit and healthy with no history of VTE, but who was taking a second-generation combined oral contraceptive pill (COCP). She developed acute onset of pleuritic chest pain and borderline tachycardia, but was stable with regard to blood pressure and oxygen saturations. A CT scan showed lobar PE and her right ventricle/left ventricle ratio was normal.
Q: Is this a provoked or an unprovoked event and how long would you continue anticoagulation?
On balance, the audience agreed this to be a provoked event, presumably because of the use of the COCP. However, this is a controversial area and many would consider this to be a ‘minimally provoked’ VTE, i.e. the patient is relatively low-risk, implying that some other underlying condition is increasing her overall risk. The recurrence rate for PE is high at about 30% within 5 years for most individuals with an unprovoked event, so this issue is important for clinical decision-making. The PADIS-PE22 study suggests that if anticoagulation is withdrawn, even after 18 months, recurrence rates catch up very quickly. If the unprovoked patient is not on indefinite anticoagulation, there remains an increased likelihood of recurrence.
The Importance of Patient Preference
One audience member advocated the importance of patient preference in decision-making about ongoing anticoagulation, particularly where the clinical evidence is unclear. Patients may have strong views, or their fears about quality of life, recurrence, and bleeds may influence their preference. Dr Hughes and Dr Jiménez agreed that this was an important part of the informed consent process, but noted that it should not override good, evidence-based practice.
Risk Assessment in Women, Especially Those on Oral Contraception
Prediction scores are many and varied, but are consistent in suggesting that most, if not all, men need to go on long-term anticoagulation if they present with symptoms. However, there is controversy around risk assessment and duration of therapy in women, particularly in relation to oral contraceptive use, and this dichotomy is reflected in the different prediction models in use.24 Rodger et al.25 concluded that low-risk women rated by HERDOO2, which rates hormone-related index events as unprovoked, could safely discontinue anticoagulation. Based on recent evidence that oestrogen-treated women who have had a PE or DVT are at very low risk, Dr Hughes treats the pill as a transient risk factor.
Q: Can D-dimer be used to aid decision-making about extending anticoagulation and when should this be tested?
Audience consensus was to test D-dimer 2 weeks after cessation of anticoagulation. Dr Hughes rarely tests for D-dimer in clinical practice but would consider it in younger women who are on oral contraceptives; based on warfarin data, he would test 3 weeks after cessation of anticoagulation.








