Abstract
Cardiogenic shock (CS), a state of inadequate tissue perfusion due to cardiac dysfunction, remains the leading cause of death following acute myocardial infarction (AMI). While the prognosis of CS post-AMI has improved in recent decades due to advances in treatment modalities, the mortality rates remain unacceptably high (~40–50% according to recent registries and clinical trials). Current treatment strategies for this condition include early revascularisation to restore blood flow to the ischaemic myocardium, the use of fluids and vasopressor or inotropic agents to reinstate haemodynamic parameters, and initiation of intra-aortic balloon counterpulsation (IABP) systems and active assist devices to maintain circulation. However, there is little evidence that these treatments actually improve survival rates. Even the most recent randomised trial conducted in this field (the IMPRESS trial comparing intra-aortic balloon counterpulsation to the Impella CP mechanical assist device) again failed to demonstrate any improvement in patient outcomes. The lack of evidence may reflect the relatively few randomised trials conducted in this area, likely due to difficulties in conducting such trials in an emergency setting. Moreover, most recent trials have focussed on patients in the late stages of CS, when they have become refractory to medical treatment and require mechanical circulatory support. This article reviews the available literature concerning the treatment of CS post-AMI in light of these limitations, and provides some evidence-based recommendations for best practice, including an updated treatment protocol.
INTRODUCTION
Cardiogenic shock (CS) is the leading cause of mortality following acute myocardial infarction (AMI). It is defined as a physiological state of end-organ hypoperfusion due to reduced cardiac output, which can ultimately irreversibly damage vital organs.1 CS has a wide clinical spectrum, ranging from preshock (hypoperfusion without hypotension, at risk of developing CS) to refractory CS (unresponsive to conventional therapies).2 Recognising the signs of preshock (e.g. a fall in urine output and rapid heart rate) is critical for early intervention and preventing progression to refractory CS.2
Available interventions for CS include fluid boluses (to maintain cerebral perfusion), ventilatory support, revascularisation, pharmacological treatment (e.g. vasopressors and inotropes), and mechanical circulatory support (MCS). Unfortunately, despite available treatment modalities, the mortality rates of CS post-AMI still approach 40–50%;3 those with refractory CS have the worst prognosis.2
Current treatment guidelines for CS post-AMI are mostly based on individual experiences, case series, or registries due to limited evidence from prospectively randomised clinical trials (RCT). Moreover, the RCT conducted so far provide little evidence that, beyond early revascularisation (ERV), the available interventions have any relevant effect on reducing mortality rates in patients with CS post-AMI. The lack of efficacy may be because RCT have traditionally only altered one element in the recommended treatment protocol at a time, which may not be sufficient to bring about improvements in mortality post-AMI. In addition, most RCT have focussed on the late stages of CS, when patients already require MCS. Considering these limitations, the available literature on the treatment of CS post-AMI was reviewed, with the aim of revising current treatment practices.
CURRENT TREATMENT PARADIGM FOR CARDIOGENIC SHOCK POST-ACUTE MYOCARDIAL INFARCTION
The classic clinical syndrome of CS is diagnosed as hypotension (systemic blood pressure <90 mmHg for 30 min or mean arterial pressure <65 mmHg for 30 min or vasopressors required to achieve blood pressure ≥90 mmHg) with reduced cardiac index (<1.8 L/min/m2 or <2 L/min/m2 with support), despite having adequate or elevated left ventricular (LV) filling pressures, with signs of impaired organ perfusion (i.e. altered mental status, cold/clammy skin, oliguria, and/or increased serum lactate).4 The current treatment strategy for patients with CS post-AMI, based on European and American guidelines and experts’ recommendations,4-7 is summarised in Figure 1. In brief, following diagnosis of CS, coronary angiography is recommended to detect any acute coronary occlusion and restore coronary blood flow.4-6 Medical treatment with ventilatory support, intravenous fluids, and/or administration of pharmacological agents is then indicated to restore blood pressure and respiration. Subsequently, the three main treatment strategies for restoring organ perfusion and cardiac output in CS post-AMI are revascularisation, MCS (intra-aortic balloon counterpulsation [IABP], ventricular assist devices, and extracorporeal membrane oxygenation [ECMO]), and infusion of vasopressors/inotropes.
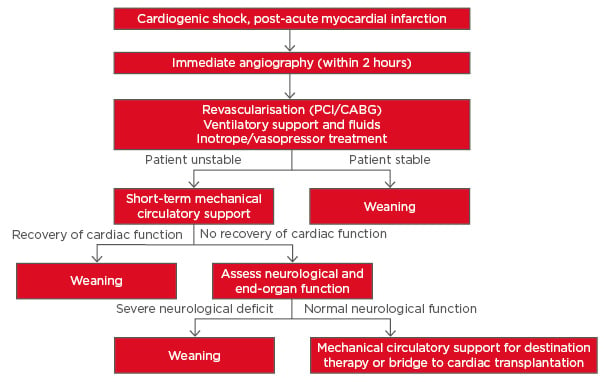
Figure 1: Current treatment strategy for cardiogenic shock post-acute myocardial infarction as outlined in the 2014 European Guidelines.6
CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention.
Adapted from Thiele et al.7
Revascularisation in Cardiogenic Shock Post-Acute Myocardial Infarction
Restoration of coronary blood flow (revascularisation) is typically achieved via primary percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). However, for patients in whom PCI or CABG cannot be performed immediately (within 2 hours), thrombolytic therapy (TT) may be considered.4,6
ERV was flagged as the most important treatment strategy for CS post-AMI in the randomised SHOCK trial (Table 1).8,9 In the emergency ERV group (n=152), revascularisation (via PCI or CABG) was initiated within 6 hours after randomisation, whereas patients in the initial medical stabilisation (IMS) group (n=150) underwent delayed revascularisation (54 hours post-randomisation on average). While mortality after 30 days did not differ significantly between the two groups (46.7% in the ERV group versus 56% in the IMS group), ERV was associated with sustained benefit compared to IMS at 6 months (50.3% versus 63.1%; p=0.027) and at 6 years.8,10 A similar benefit of early revascularisation was noted in the SMASH trial.11
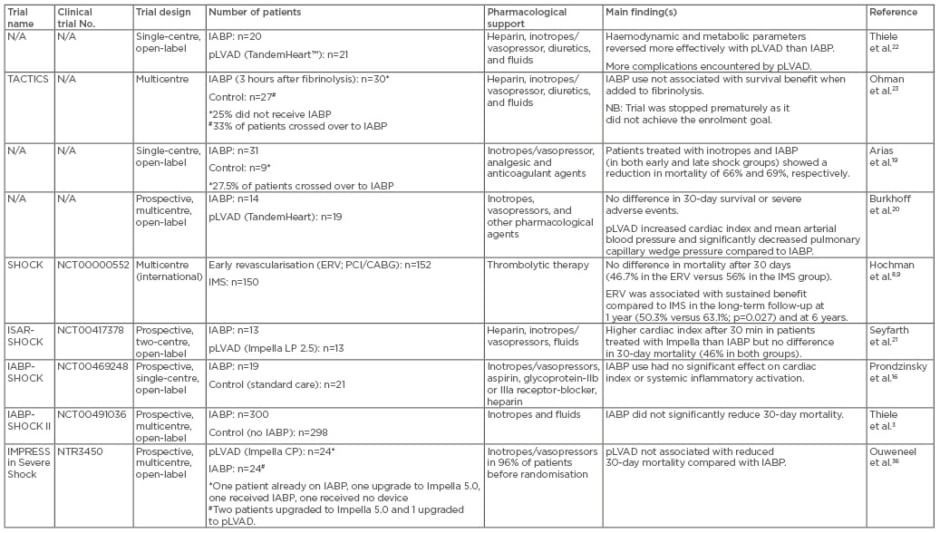
Table 1: Summary of randomised clinical trials for treatment of patients with cardiogenic shock after acute myocardial infarction.
CABG: coronary artery bypass grafting; ERV: emergency early revascularisation; IMS: initial medical stabilisation; IABP: intraaortic balloon pump; pLVAD: percutaneous left ventricular assist device; PCI: percutaneous coronary intervention.
Although not widely practiced, current guidelines encourage PCI of critical stenosis or unstable lesions in addition to the culprit lesion (Class IIa Level B recommendation in the European guidelines).6 The effect of culprit lesion-only PCI compared to multi-vessel PCI on mortality in patients with CS post-AMI is currently being explored in the CULPRIT SHOCK trial.12
Intra-Aortic Balloon Counterpulsation
Because of its low cost and relatively easy insertion/removal, IABP remains a frequently used MCS device.13 In patients with CS post-AMI, IABP increases the myocardial oxygen demand ratio (by increasing coronary and systemic circulation via diastolic augmentation) while it lowers the afterload to systolic ejection, and, therefore, increases cardiac output.14
Encouraging clinical observations of the benefit of IABP were first demonstrated in a prospective study using the abovementioned SHOCK trial patient registry.15 In this study, 856 patients with CS were treated with either IABP only (n=279), TT only (n=132), TT and IABP (n=160), or no TT or IABP (n=285). In-hospital mortality among the four groups was significantly different: TT and IABP (47%), IABP only (52%), TT only (63%), no TT or IABP (77%). The authors concluded that IABP combined with revascularisation (PCI/CABG) lowered in-hospital mortality rates compared with standard medical therapy; however, in cases where early revascularisation is not possible, TT and IABP should be initiated, followed by immediate transfer to a hospital with PCI/CABG capabilities. This study was somewhat limited by selection bias, as patients selected for IABP/TT were a lower-risk group who may have been preselected for a more favourable outcome (e.g. younger age, fewer comorbidities). Therefore, a RCT was warranted to confirm the benefits of IABP.
The first RCT to examine whether IABP in addition to PCI ameliorates multi-organ dysfunction syndrome in patients with CS post-AMI was the IABP-SHOCK trial (Table 1).16 Among the 45 patients included, no significant difference was observed between those receiving IABP and those receiving standard care with respect to severity of illness, cardiac index, or systemic inflammatory activation.16 Moreover, additional IABP treatment did not result in significant haemodynamic improvement compared with medical therapy alone.17 However, there were some limitations of this study, including discrepancies among patients within the groups.16
Following the IABP-SHOCK trial, a systematic review and meta-analysis of the literature published up to 2011 confirmed that IABP did not improve survival.18 However, the results of this meta-analysis were limited by several issues including the small number of available trials (only seven eligible studies3,16,19-23), insufficient sample sizes, and heterogeneity of included patients at baseline.18 There was also frequent crossover in several trials, and different durations of IABP support were used (ranging from 26±19–84±54 hours).16,18-20 These limitations made it difficult for any definitive conclusions to be drawn. The IABP SHOCK II trial3 (Table 1) was the first large prospective, randomised, open-label, multicentre trial, to investigate the influence of IABP support on mortality. Following presentation, patients with CS were randomly assigned to either IABP (n=300) or conventional treatment (n=298). The SHOCK II trial proved IABP is a safe therapy and offers haemodynamic benefits in CS.3 However, in terms of the primary endpoint of 30-day mortality, there was no significant difference between the two treatment groups (39.7% for IABP versus 41.3% for control; p=0.69). The 12-month follow-up data confirmed there was no significant difference in mortality rates between the two treatment groups (52% for IABP versus 51% for control; p=0.91).24 Moreover, in the survivors, quality-of-life measures (mobility, pain, depression, and others) were similar between the two treatment groups.24
The negative results of the SHOCK II trial were somewhat controversial, given IABP has been used in CS for several decades. However, the lack of benefit may reflect several limitations of the study. For example, 10% of the conventional treatment (no IABP) group crossed over into the IABP group and some patients in the control group received another MCS device.25 In addition, the timing of the IABP insertion was not standardised and was left to the discretion of the operator (IABP was inserted pre-PCI in 13.4% of cases).3 Indeed, IABP insertion after PCI was previously shown to be associated with increased in-hospital mortality.26 Furthermore, the trial was not sufficiently powered to assess a difference in mortality: given the observed mortality rate of ˜40%, >900 patients would have been required to detect the effect compared to the 600 enrolled.27 Nevertheless, as a consequence of the SHOCK II trial, IABP was downgraded to a Class IIa Level B recommendation, Level of Evidence B (can be considered in patients who do not quickly stabilise with pharmacological therapy) in the current American guidelines.5 Furthermore, IABP is not routinely recommended in the latest European guidelines (Class III recommendation, Level of Evidence B) but may be used as an adjunct for patients with mechanical complications as a bridge to surgery.28
A recent advance in IABP has been the use of a larger capacity 50 cc IAB, which showed greater systolic unloading and diastolic augmentation compared to standard 40 cc IABP in a real-world clinical study.13 The use of the large-volume 50 cc IABP as a first-line MCS strategy (with escalation when needed) was retrospectively analysed in a single-centre observational study of 100 patients with CS, with a promising rate of overall survival to hospital discharge of 66%.29 However, a RCT is needed to further validate these findings.
Percutaneous Left Ventricular Assist Devices
Percutaneous left ventricular assist devices (pLVAD) represent a newer type of MCS, and are used as a bridge to recovery or transplant, or as permanent (destination) therapy. Currently available pLVADs include TandemHeart™ (Cardiac Assist, Pittsburgh, Pennsylvania, USA); Impella® 2.5, Impella® 5.0(needs to be surgically implanted), and Impella® CP systems (Abiomed Europe, Aachen, Germany); and the paracorporeal pulsatile device iVAC 2L® (PulseCath BV, Arnhem, Netherlands).30 TandemHeart and Impella have shown improved short-term haemodynamic support; however, trials have yet to be conducted using iVAC2L.
Thiele et al.22 were among the first to report that patients with CS post-AMI who were randomised to receive pLVAD with TandemHeart (n=21) more rapidly showed improved haemodynamic and metabolic parameters than those receiving standard treatment with IABP (n=20; Table 1).22 However, pLVAD resulted in more complications, including bleeding/vascular complications.22,31-33 With regard to Impella, Seyfarth et al.21 reported it was safe to use and provided superior haemodynamic support at 30 min, but showed similar haemodynamic results to IABP at all other time points in a RCT involving 26 patients with CS (ISAR-SHOCK,21 Table 1). Although the study by Seyfarth et al.21 showed a higher cardiac index in patients treated with Impella compared to IABP, the overall mortality was similar (46%) in both groups.21 However, the small RCT by Thiele et al.22 and Seyfarth et al.21 lacked enough power to show any differences in clinical outcomes.18
A subsequent meta-analysis indicated that although pLVAD may provide superior haemodynamic support in CS compared to IABP, it does not improve early survival.34 As further evidence, a registry comparing Impella 2.5 to IABP in the setting of post-resuscitation shock reported similar mortality: the mortality rate was 77% among 35 patients treated with Impella and 79% in 43 patients treated with IABP.35 More recently, a single-centre RCT (IMPRESS, Table 1) compared IABP (n=24) and Impella CP (n=24) in patients with severe CS post-AMI, and found Impella CP did not reduce 30-day mortality compared to IABP.36 Mortality was 50% in patients treated with IABP and 46% in those treated with pLVAD (hazard ratio [HR] with pLVAD: 0.96; 95% confidence interval [CI]: 0.42–2.18; p=0.92).36 Moreover, at 6 months, mortality rates for both Impella CP and IABP were 50% (HR: 1.04; 95% CI: 0.47–2.32; p=0.923).36 Bleeding occurred more often in Impella-treated patients than in IABP-treated patients. The authors concluded pLVAD shows no additional benefit over IABP in CS post-AMI.36 However, it must be noted that the patients included in this study were in the end-stage of disease when they required mechanical support. Additionally, this study had a small sample size and was underpowered to show clinical outcome. In addition, some crossovers and upgrades occurred in this trial, at the discretion of the investigator, which may have affected the results. Moreover, the IMPRESS trial did not compare Impella to standard treatment, which is now being investigated in a Danish multicentre trial (DanShock).37
Currently, Impella is recommended in patients with severe LV dysfunction, and is approved by the US Food and Drug Administration (FDA) to provide short-term circulatory support for ≤6 hours; in Europe, it may be used for ≤5 days.38 Abiomed’s instruction for the use of Impella 2.5 L and the CP device for CS is ≤4 days and during high-risk PCI it is <6 hours.39 In addition to pLVAD, the use of ECMO should be considered for concomitant hypoxaemia.
Extracorporeal Membrane Oxygenation
ECMO, also known as extracorporeal life support, is a reliable method for urgent resuscitation in severe refractory CS and during transportation.40 While ECMO decompresses the venous system, provides flow, and ensures oxygenation, it cannot unload the left ventricle like pLVADs.41 Therefore, ECMO alone may cause progressive distention, and, subsequently, further LV failure. To address this, a recent case report showed the combined use of ECMO and Impella 5.0 could effectively manage CS post-AMI to prevent multi-organ damage as a bridge to transplantation.42 However, to date, no large RCT has been conducted on the use of ECMO in CS post-AMI.
Management of Haemodynamic Parameters with Vasopressor and Inotropic Agents
Irrespective of early revascularisation by PCI/CABG with or without MCS, volume expansion with vasopressors and/or inotropes remains a cornerstone of haemodynamic support in CS.4,7 However, excessive increases in volume and systemic vascular resistance may increase cardiac afterload and worsen cardiac failure. Indeed, according to a recent Cochrane review, neither inotropic nor vasopressor therapy reduced mortality rates in CS post-AMI.43 Instead, these agents were associated with an increase in clinically relevant arrhythmia. Inotropic and/or vasopressor therapy may also induce myocardial ischaemia, and, in some cases, even result in hypotension.44 Moreover, inotrope use has been shown to increase the mortality risk by ≤80%.45 Therefore, it appears that these drugs worsen the ischaemia-related imbalance of oxygen supply and demand in AMI.
When considering the type of vasopressor/inotrope to use, dopamine was previously recommended for use as a first-line vasopressor agent for CS post-AMI; however, RCT evidence suggests that norepinephrine is safer in this setting.46 Meanwhile, epinephrine was associated with increased 90-day mortality and worsening of cardiac and renal injury in the multinational CardShock study, which analysed the use of vasopressors and inotropes in 219 patients with CS.47 This study also found levosimendan combined with norepinephrine, as well as dobutamine combined with norepinephrine, had better outcomes, and may be a preferable treatment for CS.47 Nevertheless, more RCT are required to confirm these results, such as the OptimaCC study.48
Another trial is currently evaluating the efficacy and safety of intravenous epinephrine infusion as an early and fast haemodynamic stabiliser, associated with tight tissue perfusion monitoring, in the context of a stepwise progression in the treatment of CS, including MCS.49 At present, however, there is no clear evidence for recommendation of the use of vasopressors/ inotropes in CS, although it is advised that if they are implemented, the lowest dose possible should be used and patients should be weaned as soon as possible.5,6
RETHINKING THE TREATMENT STRATEGY FOR PATIENTS WITH ACUTE MYOCARDIAL INFARCTION COMPLICATED BY CARDIOGENIC SHOCK
As vasopressors and inotropes may worsen cardiac and renal injury, their use may be negating the benefits of MCS-related trials. As evidence, a recent study demonstrated that norepinephrine had a negative effect on the response to IABP-related microflow improvement in CS.50 So, should the use of vasopressors/inotropes in the treatment of CS post-AMI be reconsidered? And if so, what would be the best alternative?
A recent multivariate analysis showed implementation of MCS before PCI (p=0.04) and before requiring inotropes/vasopressors (p=0.05) was associated with increased survival.51 Survival was 66% when MCS was initiated <1.25 hours from shock onset, 37% when initiated within 1.25–4.25 hours, and 26% when initiated after 4.25 hours (p=0.017).51 In addition, survival was 68%, 46%, 35%, 35%, and 26% for patients requiring 0, 1, 2, 3, and ≥4 inotropes before MCS support, respectively.51 So early MCS prior to PCI or vasopressor/inotrope therapy seems to be an attractive option, but does IABP or pLVAD provide the best outcome?
Some reports have demonstrated improved survival in patients who received IABP before primary PCI compared to post-PCI (i.e. mortality rates of 25% versus 55%, respectively).26 Indeed, as IABP only provides minor circulatory support and requires a certain level of residual LV function, implementing it too late in the treatment timeline may be useless. Clinical data from the USpella registry also indicate that mortality is reduced in patients supported by pLVAD (Impella) prior to PCI.52 However, current RCT indicate pLVAD is associated with increased vascular/bleeding complications,22,31-33 and has higher associated costs compared to IABP.53
While many standardised treatment protocols for managing CS post-AMI have been established, the use of these tools still varies across centres. Based on current evidence, a new treatment algorithm is proposed (summarised in Figure 2). From this algorithm, a more uniform patient management strategy could potentially be developed for implementation in multiple centres worldwide.
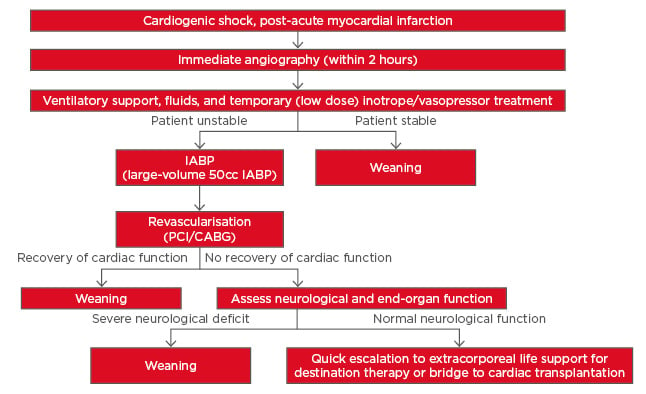
Figure 2: Proposed new treatment strategy for cardiogenic shock post-acute myocardial infarction based on currently available literature.
CABG: coronary artery bypass grafting; IABP: intra-aortic balloon pump; PCI: percutaneous coronary intervention.
In the treatment strategy proposed here, IABP (specifically using the higher efficacy 50 cc IAB) is now recommended as the first-line therapy and should be initiated prior to PCI, although this recommendation would require additional guidelines to be developed for early identification of patients for whom IABP is likely to be sufficient. Another recommendation is that low dose (at most) inotropes/vasopressors should be used only as temporary measures until IABP can be initiated prior to PCI; this may improve survival rates while avoiding the mortality associated with inotropes and vasopressor agents.54 Finally, if patients fail to stabilise, it is recommended that a quick escalation to full support using ECMO should occur. However, adequately powered RCT are required to validate the new treatment strategy.
CONCLUSIONS AND THOUGHTS FOR THE FUTURE
CS post-AMI remains challenging to treat and high mortality rates warrant further RCT in this setting. To do this, we must first overcome the inherent difficulties in conducting such trials in the emergency setting of AMI complicated by CS, as outlined previously.55 Future studies should also investigate using the available interventions at earlier stages of the disease pathology. Nevertheless, the available literature strongly indicates that it is time to reassess the standard treatment protocols for AMI patients suffering CS.








