Abstract
Anaemia is a huge global health challenge. Iron deficiency (ID) is the most prevalent, preventable, and treatable cause of anaemia worldwide. ID anaemia (IDA) is frequent in patients with heart failure. ID is an important factor in the development of heart failure but is also considered a separate condition with unfavourable clinical and prognostic consequences. In this review, the authors narrate how IDA affects the myocardium, and the possible mechanisms surrounding this impact are described. The review summarises the pathological changes seen in ID cardiomyopathy via ECG, videography, and laboratory tests. Using these tests, the early changes in the myocardium of patients with IDA have been recognised, resulting in the identification of pivotal and developmental targets for improving the morbidity and mortality of patients with IDA. Some of the progress in treatment of IDA patients has also been described. Although IDA patients experience myocardium remodelling, patients can recover heart function through iron supplementation, such as using ferric carboxymaltose. In addition, this paper includes a discussion surrounding the sex differences of the disease; however, research on this aspect is limited and should form the focus of future investigations. The authors focus on myocardial changes in adults with acute or chronic IDA.
BACKGROUND
Anaemia is a significant challenge in global health; it is characterised by lower than normal levels of red blood cells and haemoglobin per unit volume of blood and is mainly caused by genetic traits, malnutrition, and loss of trace elements. The main types of anaemia identified by studies of the global burden of diseases are iron deficiency anaemias (IDA), thalassaemias, sickle cell disease, and infection-related anaemias.1,2 Among the main causes, iron deficiency (ID) is the most prevalent, preventable, and treatable cause of anaemia worldwide.3 IDA is a condition in which iron stores in the body are insufficient to meet the needs of the patient to produce red blood cells, resulting in a lower than normal red blood cell count.
ID is the most common nutritional deficiency in various regions around the world. IDA accounts for 50% of all anaemia cases worldwide,4 making it the most acknowledged consequence of persisting ID. Moreover, anaemia has been confirmed as one of the top 20 risk factors associated with the global burden of disease by the World Health Organization (WHO).5
METHODS
For this review, the authors applied a search strategy using a combination of the keywords ‘anemias’, ‘systematic review’, ‘heart’, ‘IDA’, and ‘cardiomyopathy’. No language restrictions were applied and publications before March 2018 were screened.
The initial round of paper selection included both meta-analyses and individual studies on IDA with information on cardiomyopathy, as well as any general review for further studies containing these relationships. The present review focusses on myocardial changes in adults with acute or chronic IDA and excluded myocardial changes in IDA in infants and children.
DEFINITION
The diagnosis of IDA is defined as a haemoglobin level <12 g/dL in women and <13 g/dL in men; serum ferritin level <12 ng/mL; and mean corpuscular volume and mean corpuscular haemoglobin concentration values below the normal range (i.e., 32–36 g/dL for mean corpuscular haemoglobin concentration).6
Adequate iron reserves are required for many important processes in the body, including oxygen transport as part of the haemoglobin molecule, enzymatic reactions in the cytochrome system, and electron transport and energy metabolism in the body.7 Serum ferritin levels may also be a significant determinant of myocardial ischaemia burden during an ischaemic attack.8 ID in the adult general population is defined by the WHO as plasma ferritin <15 mg/L. In chronic heart failure, the criteria for diagnosing ID are not uniform but generally include an absolute ferritin level <100 μg/L and a functional ferritin level 100–300 μg/L in combination with a transferrin saturation <20%.9-11 Using these criteria, a study published in 2010 showed that ID was present in 37% of all patients with heart failure.12
Recent research has shown that male patients with anaemia have a higher incidence of cardiovascular diseases and a worse life expectancy than female patients with anaemia,13 which was partially due to the younger age of female patients with anaemia in this study. Among young people, the prevalence of anaemia in women is higher than in men, mainly due to the high prevalence of microcytic anaemia in women.14 In addition, anaemia may influence the mortality in patients of both sexes with chronic heart failure, but this effect is reduced in women because of a sex difference in the oxygen affinity of haemoglobin.15
SIGNS AND SYMPTOMS
Individuals with anaemia generally have a significantly decreased ability to work and reduced quality of life. They often experience fatigue, dizziness, headache, pica, palpitations, shortness of breath, and an increased heart rate.16
The initial signs and symptoms of anaemia are caused by tissue hypoxia and physiological compensatory mechanisms. Anaemia can cause left ventricular dilation, systolic dysfunction, diastolic dysfunction, and even chronic heart failure.17-19 Anaemia has been recognised as one of the independent risk factors for heart failure,20 and it has been confirmed that IDA is strongly associated with heart failure.11 Recent data have shown that patients with chronic heart failure complicated with ID have a higher rate of hospital readmission compared to patients without ID.2 One study found that up to 73% of patients with advanced heart failure also had IDA, which was confirmed by bone marrow biopsy.21 In nonischaemic cardiac failure, IDA is a well-defined adverse prognostic factor.4,12,22,23 These study findings indicate that IDA plays a significant role in the function of the heart. In addition, the myocardium is a key factor in the pathophysiology of heart failure.24,25
Anaemia and ID are common causes of heart failure, both separately and in combination.22 Honda et al.14 showed that anaemia was an independent predictor of myocardial damage or subclinical myocardial damage and cardiovascular mortality in the general population. Current evidence suggests that ID and anaemia are more prevalent in patients with heart failure and reduced ejection fraction, as well as in those with heart failure and preserved ejection fraction.26 Cho et al.27 reported that anaemia was associated with larger cardiac chambers, increased left ventricle (LV) mass, and higher LV filling pressure. The oral administration and intravenous injection of iron can help to improve the quality of life of patients with heart failure.28-30 Paying attention to early myocardial motion changes in patients with IDA can help clinicians and researchers to understand the changes in ejection fraction and ventricular function.
MECHANISMS OF IRON DEFICIENCY ANAEMIA CARDIOMYOPATHY
Anaemia is one of the many consequences of long-term ID and compensatory changes occur in the circulatory system. Researchers have long been concerned about the effect of ID on cardiomyopathy. Patients with IDA develop changes in levels of cardiac muscle as well as remodelling of cardiac muscle molecules.31 In the early stages, the cardiac output increases and forms a high-power cycle. Long-lasting hyperdynamic circulation increases the load on the heart, causing myocardial ischaemia and hypoxia, and may cause ventricular hypertrophy. If IDA is not corrected, the myocardium cannot withstand the high workload, leading to contraction and fatigue, cardiac remodelling, expansion of the ventricles, normal or thin wall thickness, and, eventually, heart failure.32 Turner et al.33 induced ID in mice and found that the increased cardiac output and sympathetic activation resulted in left ventricular hypertrophy. Yokusoglu et al.34 demonstrated an altered autonomic balance in patients with IDA. Anaemia may cause abnormalities in sympathetic nerve activity through the perception of hypoxia in the carotid body. It is now believed that this hypoxia-linked inhibition of the mitochondrial respiratory chain or potassium channels leads to intracellular calcium accumulation, which, in turn, can lead to myocyte dysfunction. The relationship between ID and the development of left ventricular hypertrophy has been verified by several studies.33,35-37
Naito et al.38 described how increased erythropoietin concentration and cardiac STAT3 phosphorylation were associated with the compensatory, beneficial cardiac remodelling associated with chronic IDA; following this, they speculated that a corresponding decrease in these factors might initiate cardiac dysfunction in long-term IDA. This experiment also showed that progressive cardiac fibrosis is a result of ID in an animal model. In a mouse experiment, erythropoietin receptor signalling played an important role in cardiac remodelling following chronic ID through the p53 pathway.39 Dong et al.37 reported left ventricular hypertrophy and dilatation in ID rats due to mitochondrial swelling and irregular sarcomere organisation, increased mitochondrial cytochrome c release, and reactive nitrogen species in cardiomyocytes.
Iron is an essential element in the synthesis of collagen,40 which is an important component of the cardiovascular system due to its role in supporting and sustaining the hardness of the vascular wall. In ID, the content of collagen in heart tissue is decreased,41 which results in the decreased elasticity of the myocardium and vascular wall, as well as changes in the normal pressure–volume relationship. Iron is also an important component of the enzymatic system of cardiomyocytes. Without iron, the biological enzyme system is destroyed, and mitochondrial cytochrome c release is increased. This condition is often accompanied by mitochondrial swelling, irregular deformation of sarcomeres, and increased presence of reactive nitrogen species in cardiomyocytes, eventually leading to muscle cell damage.42 IDA increases the production of brain natriuretic peptide and oxygen free radicals, increases platelet aggregation, accelerates atherosclerosis progression, and increases the risk of thrombosis in coronary arteries with underlying lesions, causing coronary artery occlusion.43 In addition, ID promotes lipid peroxidation and increases the levels of endothelial cells and reactive oxygen species.44 Increased oxidative stress counteracts the vascular endothelial cells and impairs their function.45
DIAGNOSTIC STUDIES
In ongoing IDA, the patient develops cardiac hypertrophy and cardiac chamber enlargement, eventually leading to heart failure.38 Various auxiliary examinations can be used to identify changes in the myocardium of IDA patients. ECG have been used to show that IDA may be associated with prolonged P-wave duration and dispersion.19 In an IDA study of pregnant women in their second trimester, a significant decrease in QRS duration and increase in QTc were observed.46 Another group of researchers observed a shortened QTc in nonpregnant women with severe IDA. The authors believed that this finding was due to hyperactivity of the sympathetic nerves secondary to the hyperdynamic circulation.47 T-wave abnormalities, including flat and negative T-waves in lead II, III, avF, and V2–V4, were more frequent in these patients and 90% of subjects in the study group had tachycardia and ECG abnormalities. There was a negative correlation between haemoglobin and serum ferritin levels, and tachycardia and electrocardiogram abnormalities.46
A number of studies have used echocardiography to confirm changes in the myocardium. Two-dimensional echocardiography has revealed changes in the intraventricular diameter parameters. The left ventricular end-diastolic and left ventricular end-systolic diameters, left ventricular posterior wall thickness, and left ventricular mass index were significantly elevated in patients with anaemia.27 In addition, the LV ejection fraction did not differ significantly between groups and the left atrial volume index was increased in patients with anaemia.27 Doppler measurements have shown that the peak E velocity (Ve), peak A velocity (Va), ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity (E/E’ ratio), stroke volume, and cardiac index were elevated significantly in the anaemia group. There was no significant difference in the deceleration time of mitral inflow velocity, peak E’ velocity, or peak A’ velocity.27 Goncharova and Govorin3 reported alterations in diastolic function in the majority of LV segments with myocardial tissue, including decreased Ve, increased Va, and decreased Ve/Va during the dynamic observation of patients with severe IDA. In a previous study,48 the global longitudinal strain, global area strain, global radial strain, and global circumferential strain of the LV by three-dimensional speckle-tracking echocardiography were measured. Results showed LV remodelling and LV systolic dysfunction in patients with haemoglobin levels in the range of 6–9 g/dL.48 In another study, researchers evaluated the left atrium (LA) function in patients with IDA using two-dimensional speckle-tracking echocardiography (Figure 1).49 The new LA function parameters, global peak atrial longitudinal strain, and strain rate of the systolic LV, as well as the early and late diastolic LV strain rate curves of the LA, exerted better potential for the accurate assessment of LA dysfunction in patients with IDA.49 The left atrial reservoir function and pipeline function have been shown to decline, while the booster pump function has been shown to increase.49
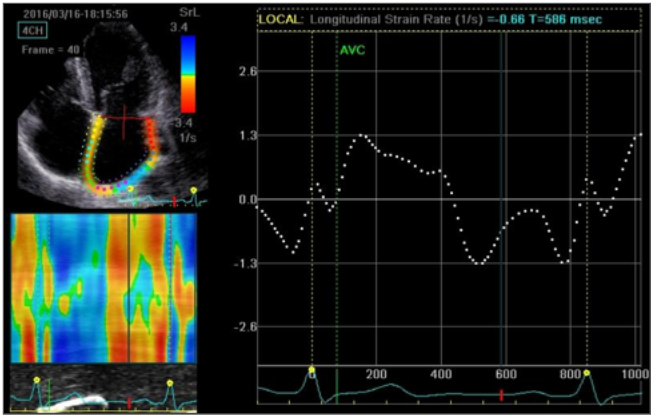
Figure 1: Global longitudinal left atrial strain rate from an apical four-chamber view of a severe iron deficiency anaemia patient.
Nagao et al.50 demonstrated the robust relationship between myocardial ID and nonischaemic heart failure by T2* cardiac MRI. Kurisu et al.51 assessed the effects of haemoglobin level on the myocardial washout rate of thallium-201 (Tl-201) in patients with normal myocardial perfusion assessed by myocardial perfusion single-photon emission CT (SPECT). The haemoglobin level was inversely associated with myocardial washout rate of Tl-201 in patients with normal myocardial perfusion on SPECT. Furthermore, the myocardial washout rate of Tl-201 was in accordance with haemoglobin-regulated coronary blood flow.51 These findings provide a new way to reveal the effects of anaemia on the myocardium.
Bellotto et al.52 examined the relationship between haemoglobin and the risk of myocardial injury, reporting increased cardiac troponin I levels in patients with anaemia secondary to upper gastrointestinal bleeding and no other clinical signs or symptoms of coronary insufficiency on enrolment. In the context of these studies, it can be inferred that myocardial injury in patients with acute or chronic IDA can be detected and monitored early by measuring troponin I levels. This indicator is more sensitive than other indicators of myocardial damage.
TREATMENT
The cardiomyopathy of IDA may be completely reversible.53 Sohn et al.54 used chest electrocardiographic and radiographic images in a 66-year-old woman with cardiac hypertrophy who developed severe chronic anaemia due to long-term bloodletting with cupping. After 3 months of iron supplementation, the patient’s cardiac hypertrophy improved remarkably. In another study, low haemoglobin level was associated with larger cardiac chambers, increased LV mass, and higher LV filling pressure.27 Appropriate correction of anaemia decreased LV mass, LA volume, and E/E’. Furthermore, ferric carboxymaltose may alleviate IDA in patients for whom oral iron supplementation is ineffective.55 A large study also found that ferric carboxymaltose was suitable for those patients who could not tolerate oral iron and effectively improved the symptoms of IDA.56
CONCLUSION
The present review focussed on several possible mechanisms for the development of IDA cardiomyopathy. IDA-related myocardial changes are caused by both ID and anaemia, as well as the combined effects of the two factors.31 The myocardial changes of IDA cardiomyopathy can be detected by echocardiography and laboratory examinations. In addition, three and two-dimensional speckle-tracking echocardiography can be used to detect early changes in the LV myocardium in patients with IDA.48,49 The laboratory index of cardiac troponin I can be used to assess myocardium damage in early IDA cardiomyopathy, which may be reversed by treatment. Ferric carboxymaltose may be a better method to supplement iron in patients with IDA. In summary, awareness of the myocardial effects in patients with IDA should be improved to help preclinical diagnosis and treatment, which have pivotal and developmental prospects in reducing morbidity and mortality. Further studies on the sex differences in the impact of IDA on cardiomyopathy are warranted.








