Abstract
Severe acute respiratory syndrome coronavirus 2, or COVID-19, has triggered an unprecedented pandemic situation across the globe. Patients with COVID-19 frequently experience a range of clinical complications driven by their health status, comorbidities, and disease responsiveness. Patients with COVID-19 also encounter cardiovascular conditions that potentially increase their risk for mortality. Few clinical studies reveal the development of ST segment elevation myocardial infarction (STEMI) in patients with COVID-19.
New York City, USA, continues to witness and report a high incidence and prevalence of COVID-19 infections. New York City’s healthcare centres and hospitals have treated more than 6,000 cases of COVID-19 pneumonia in their inpatient and intensive care units.
The authors conducted a retrospective study of patients admitted to NYC Health + Hospitals, Queens, New York City, USA, with confirmed COVID-19 reverse transcriptase-PCR test findings between 29th March 2020 and 1st May 2020. The authors used a retrospective case series design to evaluate the association between laboratory-confirmed COVID-19 infection and hospitalisation for acute myocardial infarction. They utilised a series of ECGs to record and analyse STEMI patterns across patients with COVID-19. This study aimed to determine the risk/incidence of STEMI in patients with COVID-19, and its impact on their clinical presentation, angiographic findings, and clinical outcomes. The authors hypothesised STEMI as a significant COVID-19 complication, with the potential to impact the long-term prognostic outcomes of patients with COVID-19.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) predominantly leads to COVID-19.1 The spike glycoproteins over the envelope of the positive-sense single-stranded RNA coronaviruses give them a crown-shaped appearance. The coronaviruses belong to the Coronaviridae family, Orthocoronavirinae subfamily, and order Nidovirales. The 16–140 nm size, pleiomorphic form, elliptical structure, and ability to tolerate lower temperatures of SARS-CoV-2 add to its virulence and pathogenicity. COVID-19 progresses with cough and fever symptoms; however, its clinical manifestations trigger mild respiratory illness in 80% of affected patients. More than 15% of patients with COVID-19 develop severe pneumonia and shock, requiring hospital-based care. Nearly 5% of patients with COVID-19 develop life-threatening complications, requiring medical management in intensive care units (ICU).2
Patients with SARS-CoV-2 frequently encounter cardiovascular complications, including Type 1 or 2 myocardial infarction (MI), myocardial injury, ST segment elevation, myocarditis, worsening or new-onset heart failure, cardiogenic shock, severe arrhythmias, and venous thromboembolic episodes.3 The authors aimed to evaluate the clinical presentation, angiographic findings, and clinical outcomes of STEMI in patients with COVID-19.
The authors identified six patients with COVID-19 with STEMI, who presented initially to a non-percutaneous coronary intervention (PCI)-capable hospital and were transferred to a PCI-capable facility or managed with fibrinolytics. ST segment elevation in some patients developed during their clinical presentation; however, other patients developed it during the course of their hospitalisation. Patients with COVID-19 who underwent cardiac catheterisation had a poor prognosis due to their obstructive and non-obstructive patterns. The multifactorial aetiology and pathophysiology of myocardial injury in these patients warranted further investigation to inform their diagnostic and treatment management.
METHODS
The authors present six cases in this article. Permission was obtained from the Icahn School of Medicine at Mount Sinai Institutional Review Board, New York City, USA (No.: STUDY-21-00049).
RESULTS
Case 1
A 59-year-old male with a history of hypertension arrived at the emergency department (ED) with 2 days of subjective fever and light-headedness. Reverse transcriptase (RT)-PCR testing confirmed the SARS-CoV-2 antigen. The patient encountered decompensation shortly after admission due to multiorgan dysfunction and acute hypoxic respiratory failure, requiring mechanical ventilation. An ECG on the day of admission demonstrated left ventricular hypertrophy and a dilated right ventricle (RV) that appeared hypokinetic on limited views. The left ventricle was not well visualised, but appeared normal in limited views.
The next day, the patient experienced cardiac arrest, followed by the return of spontaneous circulation. The ECG findings revealed sinus rhythm, with a rate of 71, and intraventricular conduction delay; 2 mm ST elevation in leads II, III, and augmented vector foot (aVF); 5 mm ST elevation in leads V4–6; and 2 mm ST elevation in leads I and augmented vector left (aVL), which are consistent with an acute inferolateral STEMI. The decision was then made to administer thrombolytics as the patient became haemodynamically unstable to be transferred for a primary PCI after they had a cardiac arrest. The repeat ECG (Figure 1A) showed 1 mm ST elevation in lead II and aVF, 1 mm depression in lead I and aVL, and 4 mm ST elevation in leads V4–6.
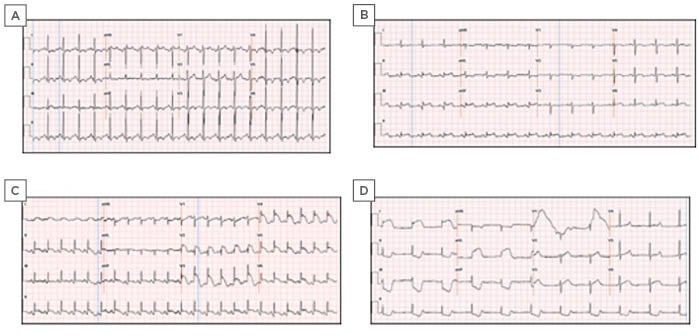
Figure 1: ECG ST segment elevation in four patients (cases 1–4) with COVID-19.
A) ECG ST segment elevation in lead II and aVF, and a 1 mm depression in lead I, aVL, and 4 mm ST segment elevation in leads V4–6. B) ECG showed ST segment elevation in leads II, III, aVF, and V4–6 and ST depressions in lead aVL. C) ST segment elevation in leads II, III, aVF, and leads V2–6. D) ECG post-intubation revealed ST segment elevations in the lateral leads, reciprocal ST segment depressions in the inferior leads, and severe sinus bradycardia competing for junctional escape.
aVF: augmented vector foot; aVL: augmented vector left.
The patient’s condition did not favour transfer as they remained haemodynamically unstable and, subsequently, the alteplase infusion was continued. The patient’s respiratory and renal functions deteriorated within a few hours of medical management, resulting in intolerable cardiac arrest, and, eventually, the patient expired.
Case 2
An 80-year-old female who was overweight and with a history of hypertension arrived at the ED with subjective fever, cough, and diarrhoea for the last 2 weeks. The patient was found to be COVID-19 positive via an RT-PCR test. The clinical findings revealed a marked elevation in inflammatory markers and bilateral multifocal pneumonia, as seen on their chest X-ray. The D-dimer was elevated to 911 ng/L. ECG on admission showed sinus tachycardia with supraventricular premature complexes and probable old inferior MI. The pro brain-type natriuretic peptide value of 5,308 pg/mL exceeded the normal reference range of 300–900 pg/mL.4
The patient was transferred to the ICU for worsening hypoxemic respiratory failure secondary to bilateral pneumonia. The patient developed a pneumothorax, requiring chest tube placement and invasive positive pressure ventilation. Repeat ECG showed ST elevations in leads II, III, aVF, and V4–6, with depressions in leads I and avL (Figure 1B). The cardiac troponin-I level of the patient increased from 0.05 to 2.16 within 4 hours of ICU admission, which exceeded the normal limit of 0.03 ng/mL.5 The patient’s instability restricted her transfer for emergency catheterisation due to a reduction in preload after intrathoracic pressure elevation.6 The subsequent ECGs after alteplase administration revealed slight improvement in persistent ST elevations.
An ECG after thrombolytics showed an ejection fraction (EF) of 60% with Grade I (mild) left ventricular diastolic dysfunction, dilated RV cavity size, and reduced RV systolic pressure. The pulmonary artery systolic pressure was recorded as 62.76 mmHg. No left ventricular wall motion abnormality was identified; however, subsequent ECGs revealed the resolution of ST elevations and inferior Q waves. The patient’s treatment course was complicated by atrial fibrillation, with a rapid ventricular response. The rate control was achieved with amiodarone; however, the patient ultimately expired within a few days of medical management.
Case 3
A 62-year-old female with a medical history of cerebral palsy and dementia was brought in by the emergency medical services from a nursing home after developing respiratory distress and hypoxia (oxygen saturation: 85% at room air). The preliminary emergency room assessment revealed fever, tachycardia, and hypoxia requiring intubation. Telemetry monitoring did not reveal any arrythmia. Cardiology was consulted in the ED for STEMI on ECG. The ECG (Figure 1C) showed 2 mm ST elevation in leads II, III, and aVF; 5 mm ST elevation in leads V2–6; and 1 mm ST elevation in lead I. The initial troponin was recorded as negative; however, neither a repeat troponin nor an initial ECG was obtained.
The COVID-19-related RT-PCR testing showed a positive result, while the D-dimer was elevated to 35,807 ng/dL (exceeding the reference range of 0.4–250.0 ng/mL). The chest X-ray showed extensive right mid–lower lung field (pulmonary) consolidation, and nonspecific left basilar markings. The patient’s transfer to another medical facility with PCI capability was also constrained due to haemodynamic instability. The patient received loading doses of aspirin and plavix; however, the patient experienced cardiac arrest and eventually expired in the ED before arriving at the ICU.
Case 4
A 71-year-old male with a history of Type 2 diabetes and hypertension was brought in by the emergency medical services with altered mental status. The patient appeared afebrile but was found to be hypoxic, with oxygen saturation levels of 85%. The chest X-ray was remarkable for bilateral patchy infiltrates. Laboratory workup revealed diabetic ketoacidosis. The patient was admitted to the ICU for further management.
The diabetic ketoacidosis was resolved in ICU; however, the patient required increasing oxygen requirements, and was intubated and placed on mechanical ventilation. The post-intubation ECG revealed ST elevations in the lateral leads with reciprocal depressions in the inferior leads, and severe sinus bradycardia competing for junctional escape (Figure 1D).
Care was escalated and arrangements were made for transferring the patient to another facility for PCI. Loading doses of aspirin and ticagrelor were administered and followed by therapeutic anticoagulation with heparin. The patient, however, experienced polymorphic ventricular tachycardia, which was seen on telemetry, and arrested before being transferred. Return of spontaneous circulation was achieved after one round of cardiopulmonary resuscitation. The patient was eventually transferred for cardiac catheterisation.
Another ECG revealed new, inferior ST elevations and the resolution of lateral ST elevations. A left heart catheterisation revealed a large proximal right coronary artery (RCA) thrombus, complete thrombotic occlusion of the right posterior descending artery, and a non-occlusive thrombus in the right precordial leads, which restricted the administration of PCI. The decision to withhold PCI relied on the concern for distal embolisation and the underlying thromboembolic process. The anticoagulation protocol was eventually continued that included aspirin, ticagrelor, and cangrelor infusion. The ECG confirmed wall motion abnormalities, including severe hypokinetic left ventricular apex, hypokinetic mid-inferior and inferolateral walls, and severe hypokinetic RV, without any evidence of pulmonary hypertension. The patient continued to decompensate with multiorgan failure and, eventually, expired on the Day 6 of hospitalisation.
Case 5
A 58-year-old male with a history of diabetes and hyperlipidaemia arrived at the ED after 4 days of subjective fever and cough. A chest X-ray revealed ill-defined bibasilar opacities suggestive of multifocal pneumonia. The COVID-19 RT-PCR testing showed a positive result and the patient developed acute onset of chest pain shortly after admission. The ECG revealed Q waves and ST elevation in inferior leads and Q wave in leads V5–6, with ST depression in augmented vector right and aVL (Figure 2A). The patient was transferred to another facility for urgent left heart catheterisation and was found to have RCA occlusion combined with emboli showering to distal territories. Three stents were placed in the RCA and aspiration thrombectomy was performed. The patient developed worsening hypoxic respiratory failure, requiring mechanical ventilation. Aspirin, clopidogrel, and atorvastatin were continued, while therapeutic anticoagulation with enoxaparin was started post-catheterisation.
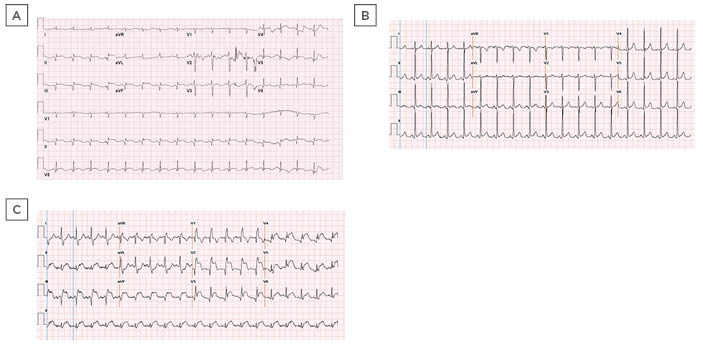
Figure 2: ECGs of two patients (Cases 5 and 6) with COVID-19.
A) ECG revealed Q waves and persistent ST elevations in the inferior leads. B) Initial ECG on presentation demonstrated sinus tachycardia. C) Repeat ECG revealed acute ST segment elevation in leads II, III, aVF, and V2–6.
aVF: augmented vector foot.
A follow-up ECG revealed Q waves and persistent ST elevations in the inferior leads. The subsequent ECG demonstrated an EF of 30%, hypokinetic mid-anterolateral and mid-inferolateral wall, severe anterior apical and apical lateral wall hypokinesis, a severely hypokinetic apical septum, and a left ventricular thrombus. The patient was transitioned to intravenous heparin and continued with aspirin and clopidogrel, based on the concern for COVID-19 related myocarditis versus stress cardiomyopathy, due to the pattern of wall motion abnormalities revealed on the ECG. However, the patient’s condition deteriorated, and they expired on the Day 10 of their hospital stay.
Case 6
A 40-year-old female, with a medical history significant for Type 2 diabetes, hypertension, and psychiatric disorder, arrived at the ED with a productive cough and shortness of breath for 6 days and severe, constant, and non-radiating retrosternal chest pain for 24 hours. The patient was admitted with the impression of acute hypoxic respiratory failure, requiring supplemental oxygen, likely secondary to COVID-19. The SARS-CoV-2 infection was confirmed via an RT-PCR.
The ECG on admission indicated sinus tachycardia (Figure 2B). The initial troponin finding was negative; however, the patient complained of worsening chest pain the following day and a repeat ECG revealed acute ST elevation in leads II, III, aVF, and V2–6, and reciprocal ST depressions in lead I and aVL (Figure 2C). The patient was loaded with aspirin, clopidogrel, and started on atorvastatin. The therapeutic anticoagulation was administered with heparin and the patient was transferred for cardiac catheterisation. A thrombotic lesion was found in the ostium of the left anterior descending artery, requiring PCI. The high thrombus burden attributed to the distal left circumflex coronary artery was treated with intracoronary alteplase.
The point of care ultrasound indicated a severely reduced EF of 10–15%, with diffuse wall motion abnormalities. The patient’s course of treatment was complicated by cardiogenic shock, requiring mechanical ventilation during the PCI procedure. The Impella® (Abiomed, Danvers, Massachusetts, USA) was subsequently placed for reversing the cardiogenic shock (cardiac index: 1.7 on pulmonary artery catheter). The patient’s clinical course, however, did not improve, and they expired 2 days later.
DISCUSSION
Several studies have highlighted a constellation of observed cardiovascular consequences that appear unique to COVID-19. The evidence of myocardial injury attributing to troponin level elevation was found to be common among patients hospitalised for COVID-19. The potential causes that trigger this troponin elevation include stress cardiomyopathy, hypoxic injury, and an ischemic injury from a systemic inflammatory response syndrome. The clinical observations confirm a wide variety of findings in patients with COVID-19 based on their ST elevations on ECG and emergent angiography status. The findings vary from classic obstructive coronary artery disease, non-obstructive coronary artery disease, normal epicardial coronary arteries, and/or left ventricular dysfunction due to myocarditis or stress-induced cardiomyopathy.7
The authors identified six patients with COVID-19 with ST segment elevation, indicating the onset of acute MI. They determined a marked variation in the baseline characteristics and clinical presentation of the included patients. They further tracked the development of severe COVID-19 manifestations that potentially complicated medical management of treated patients. Details regarding pertinent history, baseline characteristics, laboratory, and ECG findings are outlined in Table 1. The authors postulate that the symptomatology, signs, and manifestations of COVID-19 may have concealed the classic symptoms and presentations of acute MI, thereby leading to its underdiagnosis. The ECG findings concerning the selected patients revealed normal or abnormal wall motions. The COVID-19 candidates for cardiac catheterisation prevalently developed obstructive pulmonary disease. The authors’ observations further revealed marked elevations in the D-dimer levels of the treated patients.
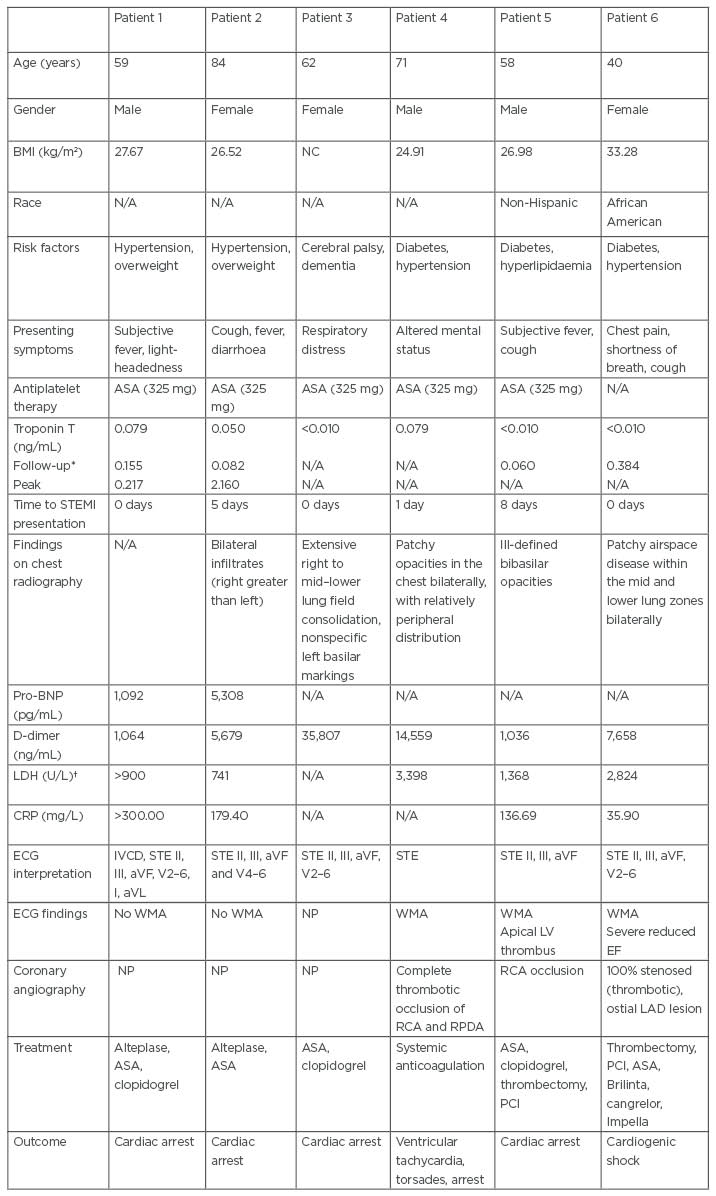
Table 1: Baseline characteristics and case details of six confirmed patients with COVID-19 and acute myocardial infarction.
*Follow-up laboratory values typically obtained within 6–12 hours of initial results.
†Reference range: 135–225 U/L.
ASA: aspirin; aVF: augmented vector foot; aVL: augmented vector left; BNP: brain-type natriuretic peptide; CRP: C-reactive protein; EF: ejection fraction; IVCD: intra ventricular conduction delay; LAD: left anterior descending; LDH: lactate dehydrogenase; LV: left ventricle; N/A: not applicable; NC: not calculated; NP: not performed; PCI: percutaneous coronary intervention; RCA: right coronary artery; RPDA: right posterior descending artery; STE: ST segment elevations; STEMI: segment elevation myocardial infarction; WMA: wall motion abnormality.
A clinical study revealed elevated D-dimer levels in patients who underwent primary PCI for STEMI. The higher D-dimer levels correlated with larger MI size, suggesting that D-dimer may be a diagnostic marker of advanced myocardial injury.8 Myocarditis has been shown to mimic acute coronary syndrome and has been associated with COVID-19 with high mortality. It is challenging to differentiate myocarditis from acute coronary syndrome in the acute setting. Endomyocardial biopsy is the gold standard for the diagnosis of myocarditis, but it is an invasive procedure that, at the peak of the pandemic, posed several limitations to the patient and the healthcare worker. The cases above who presented with dynamic ECG changes with preserved left ventricular EF were suggestive of unobstructed coronaries, raising the concern for myocarditis.
The clinicians continue to observe several plausible factors concerning cardiac injury throughout the COVID-19 pandemic. The arteriovenous thrombosis is a hallmark of severe COVID-19 infection and correlates with vascular injury and prothrombotic cytokines released due to intense systemic inflammatory responses.9 The prothrombotic and procoagulant states may potentially elevate the risk of coronary thrombosis at the sites of plaque disruption. The factors contributing to coronary thrombosis include the production of neutrophil extracellular traps (from intraplaque and circulating neutrophils), increased platelet activity, elevated accumulation of procoagulants or tissue factor, impaired fibrinolysis, and overall disruption of anticoagulant function of the endothelium.10 The clinical studies postulate cytokine storm, hypoxic injury, and coronary spasm or microthrombi as the significant factors with the potential to trigger myocardial injury in patients with COVID-19.11
An Italy-based systematic pathological analysis correlated Type 2 MI with the development of microthrombi in the coronary vasculature of patients with COVID-19. The pathologists revealed marked differences in composition between microthrombi and intramyocardial thromboemboli in subjects who were COVID-19-negative and coronary thrombi retrieved from patients with STEMI who were COVID-19-positive/negative.12 The previously reported clinical studies also highlight the impact of acute infections on the MI predisposition of patients with COVID-19. The medical literature additionally provides evidence concerning the attribution of systemic viral infections or influenza to the development of acute MI and inflammation.13 Plausible evidence correlates STEMI events with the systemic inflammatory responses triggered by COVID-19 infection.
The COVID-19-related clinical guidance by the American College of Cardiology (ACC) reveals that COVID-19 complications commensurate with SARS, Middle East respiratory syndrome, and influenza analogues. Pre-COVID-19 data regarding the influenza virus suggest that patients with acute respiratory infections are highly predisposed to atherosclerotic plaque rupture and eventual MI, under the impact of profound inflammatory responses and haemodynamic changes.14,15
The standard treatment protocol for patients with STEMI includes invasive revascularisation (within 90 minutes of hospital presentation), or fibrinolytic therapy in the absence of invasive revascularisation (after 90 minutes of hospital presentation). Fibrinolytic therapy has recently gained much attention, based on its potential to treat COVID-19-related STEMI in medical centres without cardiac catheterisation facilities. The challenges concerning medical management of STEMI in COVID-19 reciprocate with the unstable presentations of undetermined aetiologies. Furthermore, the clinical correlation between ST segment elevation and its potential causes in patients with COVID-19 appears highly challenging in emergencies. The clinical studies have yet to determine the mortality attribution of ST segment elevation in COVID-19 scenarios. Future studies also need to evaluate the risks and benefits of fibrinolytic therapy in the setting of COVID-19. The current body of evidence appears clueless regarding the MI predisposition of patients with COVID-19 following their acute or short-term post-infection period. Patients with COVID-19 experience a high predisposition for aggressive inflammatory responses that potentially increase their risk for hypercoagulability. However, future studies still need to determine the long-term prognosis of acute coronary syndrome in patients with COVID-19.
The authors’ study has several limitations. Firstly, they relied on the findings of a single hospital, which challenged the generalisability of findings across other hospital settings with cardiac catheterisation capabilities in New York City. The reporting of incomplete data in the selected case reports increased the risk of information bias to many folds.
Secondly, the authors may have missed many cases concerning ST elevation MI and inappropriate treatments due to the surge of patients in the ED. Thirdly, the ECGs were not performed on many patients periodically, despite their troponin elevation, due to the probable masking of their symptoms by concurrent respiratory failure. The awareness of these limitations could have improved the medical management and treatment outcomes of patients with COVID-19.
CONCLUSION
Exploring the significant unknowns regarding the short and long-term sequelae of COVID-19 infection among hospitalised patients is key to improving treatment outcomes. Respiratory tract symptoms mostly determine the clinical course of COVID-19 infection, yet there appears to be a unique interplay between coronavirus disease and acute coronary syndrome. The recognition of ST elevation MI as a complication of COVID-19 may inform its diagnostic monitoring, and help to determine prognostic outcomes. This case series highlights the importance of clinical surveillance and laboratory testing in the subgroup of patients with COVID-19, to analyse their atypical symptomatology, and avoid potential delays in recognising their treatment modalities. The authors determined a critical knowledge gap concerning baseline characteristics, comorbidities, revascularisation strategies, and outcomes of admitted patients with COVID-19-related ST elevation MI. Their findings advocate the standardisation of diagnostic and therapeutic protocols to enhance the medical management of patients with COVID-19 with STEMI.








