Abstract
Introduction: The coronavirus disease (COVID-19) infection is proved to be involved in the onset of thromboembolism episodes. This study aims to evaluate the prevalence of thromboembolic complications in patients with COVID-19 from March until May 2020.
Methods: A literature review was conducted in MEDLINE (via PubMed), Scopus, Embase, Cochrane, and CINHAL without any language and date of publication restriction (Prospero registration number CRD42020186925). The inclusion criteria were as following: 1) patients with diagnosis of COVID-19; 2) occurrence of thromboembolic event, and 3) patients older than 18 years of age.
A multi-variable random effects model was computed accounting for correlations among outcomes by considering a heterogeneous compound symmetry covariance matrix.
Results: Observational studies included 2,442 participants from 268 to 7,999 participants per study, 1,014 (41.52%) were male and 825 (33.78%) were female. The multi-variable pooled event rate of acute myocardial infarction was rare, estimated to be 0.03 (95% confidence interval [CI]: 0.00–0.07; p=0.23); this is also true for the meta-analytical estimate of disseminated intravascular disease which was 0.04 (95% CI: 0.00–0.08; p=0.03). Conversely, other events were found to be more frequent. Indeed, the pooled proportion of pulmonary embolism was 0.14 (95% CI: 0.08–0.20; p<0.001), while the venous thromboembolic event rate is 0.15 (95% CI: 0.09-0.30; p=0.04). The pooled intrahospital mortality rate was equal to 0.12 (95% CI: 0.08–0.16; p<0.001).
Conclusions: Thromboembolic events, particularly venous thromboembolic event rate and pulmonary embolism, are a frequent complication in patients hospitalised with COVID-19. These findings suggest that the threshold for clinical suspicion should be low to trigger prompt diagnostic testing and that evaluation of therapeutic treatment should be considered in patients in intensive care units with COVID-19.
INTRODUCTION
The novel coronavirus disease (COVID-19) has posed an unprecedented threat to global healthcare systems. The case fatality rate has been estimated to be as high as 15% in some countries.1 The reason for high mortality rates of the new coronavirus infection is still unclear.
Clinical manifestations may vary from asymptomatic patients to acute respiratory distress syndrome, shock, and multi-organ failure associated with an increased risk of death.2 Coagulation abnormalities have been reported since the start of the pandemic. Some authors supposed that these abnormalities might be similar to those reported in the previous severe acute respiratory syndrome coronavirus (SARS-CoV-2) infections. Chong et al.3 reported an incidence of 11.4% for pulmonary embolism (PE) and 20.5% for deep venous thrombosis (DVT) in SARS-CoV-2-infected patients.3 The clinical course of COVID-19 infection is often accompanied by systemic inflammation, endothelial dysfunction, and coagulation activation, which may evolve into overt disseminated intravascular coagulopathy (DIC).4 Moreover, the systemic activation of blood coagulation and pulmonary thromboinflammation with local vascular damage caused by COVID-19 may increase the risk of venous thromboembolism (VTE) and pulmonary artery thrombosis.5
From a pathogenetic point of view, the mechanism of hypercoagulability in patients with COVID-19 infection is quite clear;6 however, there are still no clear data on the incidence of those episodes in patients with COVID-19 despite the numerous reports and studies related to thromboembolic episodes.
Better understanding of COVID-19-related thromboembolic risk will help to optimise diagnostic strategies and guide the design and conduction of randomised controlled trials on thromboembolic prevention.
Thus, the aim of this meta-analysis was to estimate the prevalence of thromboembolic complications in patients admitted for COVID-19 from March until May of 2020. As the disease and its treatments are rapidly evolving, this meta-analysis represents events during the first and the beginning of the second wave of COVID-19 infection.
MATERIALS AND METHODS
Study Design
This is a systematic review and meta-analysis. The authors followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines. The study was registered in Prospero with the number CRD42020186925. Research strategy was developed according to the Population, Intervention or exposure, Comparison, and Outcomes (PICO) study design model; these factors were patients with COVID-19, thromboembolic event, none, and incidence of thromboembolic events, respectively. The research strategy was then adapted according to the specific characteristics of each database.
Data Sources and Search
The literature review was conducted in MEDLINE (via PubMed), Scopus, Embase, Cochrane and CINAHL without any language and date of publication restriction. The following search terms were used and adapted in each database with proper Boolean operator: COVID-19, severe acute respiratory syndrome coronavirus-2, SARS-CoV-2, thrombosis, thromboembolism, cerebrovascular accident, myocardial infarction, hypercoagulability, heart attack, coronary disease, ischaemic heart disease, coronary heart disease, vascular disease, and cardiovascular disorder. The reference list of the retrieved studies was also searched. The last update was made on the 7th of May 2020.
Eligibility Criteria
The inclusion criteria were as follows: 1) patients with diagnosis of COVID-19; 2) occurrence of thromboembolic event (without restrictions to the damaged organ); and 3) patients older than 18 years of age. There was no restriction in the design of the studies included. Studies evaluating the outcomes of interest postmortem were excluded.
Study Selection
As reported in Figure 1, the study selection procedure was performed according to the PRISMA guidelines.7 The software Covidence8 was used in all the phases of data collection and extraction. Two blinded reviewers screened the articles in each phase. In each stage, the disagreements between the reviewers were solved by another senior expert in the field.
Figure 1: PRISMA flow chart of retrieved articles.
Data Extraction
For each article, two reviewers extracted the following data: country, study design, author, and patient population characteristics (i.e., age, sex, comorbidities, laboratory exam results, use of invasive mechanical ventilation, use of antithrombotic medications, ward, length of stay, mortality, type of thromboembolic event occurred, and number of events).
Outcomes
The outcome of interest was the prevalence of both arterial and venous thromboembolic events in patients with COVID-19. The authors included prospective and retrospective studies, case series and case reports in order to better understand the dimension of the problem.
Quality Assessment
The risk of bias was evaluated by two independent reviewers. The Joanna Briggs Institute Critical Appraisal tools were used for quality assessment, according to the design of the study9 (e.g., prospective studies, prevalence data, case report, and case series).
Agreement
Cohen’s Kappa was used to evaluate the interrater agreement among two reviewers in the full-text selection. The percentage of agreement was 56.98%. This could be explained by the large difference experience in metanalysis between the operators. A third expert reviewer reconciliated the disagreement between operators.
Data Synthesis
A multi-variate random effects model was computed accounting for correlations among outcomes by considering a heterogeneous compound symmetry covariance matrix. The multi-variate I2 heterogeneity was also calculated following the approach proposed by Jackson et al.10 A multi-level random effect meta-regression model was used to deal with heterogeneity (I2>75%), accounting also for correlation among the outcomes. Restricted cubic spline was considered for nonlinear effects. The estimates obtained with the multi-level random effect models have been adjusted by using, as moderators, the geographic areas (Europe and China), the study design (retrospective and prospective), and the median age of the study participants.
The univariate pooled random effect meta-analysis, together with the 95% confidence interval (CI), was also estimated for comparison purposes.
The incidence of discharge pooled estimate was computed using an incidence rate random effect meta-analysis calculating the events over the person time (length of hospitalisation) for each study.
Results and study level estimates were represented in a forest plot. The publication bias has been assessed via funnel plot graphical representation. Correlation matrix was computed among outcomes event rates with a 95% CI. Only endpoints reporting more than two pairwise comparisons have been computed for the correlation matrix. The analyses were conducted using R 3.6.2,11 and rms12 and metafor13 packages.
RESULTS
The search identified 2,110 studies. After title, abstract, and full-text screening, 38 studies were included in this review. Eighteen out of 26 studies were case reports and eight were case series. The remaining studies included in the meta-analysis were observational, eight were retrospective and six were prospective (Figure 1). The studies included in the metanalysis are shown in Table 1.14-51
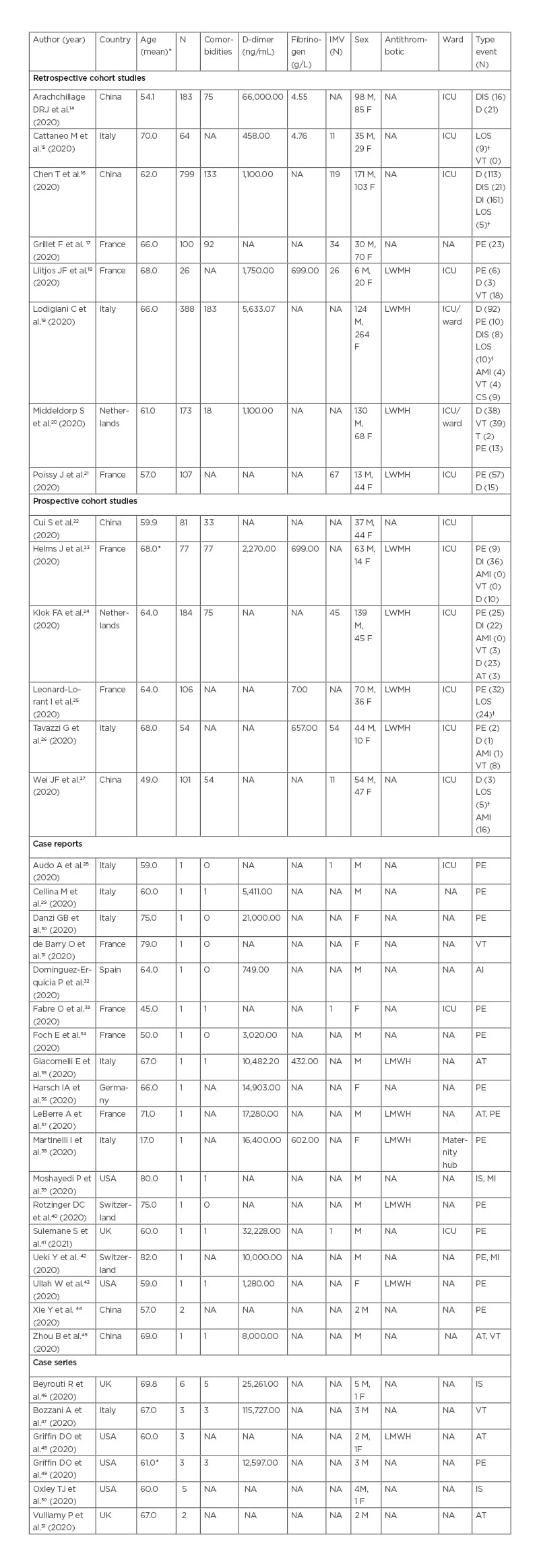
Table 1: Descriptive characteristics of retrieved studies.
*Median age
†LOS was reported as number of days
AMI: acute myocardial injury; AT: arterial thromboembolism; CS: cerebrovascular stroke; D: deceased; DI: discharge; DIS: disseminated intravascular disease; F: female; ICU; intensive care unit; IMV: intermittent mandatory ventilation; IS: ischaemic stroke; LOS: length of stay; LWMH: low-molecular weight heparin; M: male; MI: myocardial infarction; N: number of patients; NA: not applicable; PE: pulmonary embolism; T: thrombophlebitis; VT: venous thromboembolism.
Descriptive Characteristics of the Studies Included in Quantitative Analysis
Observational studies included 2,442 participants ranging from 26–79918,16 participants per study, of these 1,014 (41.52%) were male and 825 (33.78%) were female. They were mainly located in China16,22,27,52 and in France,17-19,21,23,25 the mean age reported was 49–70 years. Comorbidities were reported in 12 studies, 740 (30.30%) patients had at least one comorbidity (Table 1). Most patients included in the retrieved studies were admitted to the intensive care unit, except for those in the studies by Arachchillage et al.14 and Grillet et al.17 Antithrombotic therapy was routinely used in seven studies. They all used low-molecular weight heparin but with different dosage depending on the therapeutic or prophylactic usage. Only one study, by Llitjos et al., compared the prophylactic versus the therapeutic usage of anticoagulant18 in patients in ICU. The thromboembolic events and the number of events for each study are reported in Table 1. The considered events were grouped in the following categories: pulmonary embolism, venous thromboembolism, acute myocardial injury, arterial thromboembolism, and disseminated intravascular disease. PE was the most frequently reported event.17,20,21,23-26
Descriptive Characteristics of the Studies Included in Qualitative Analysis
Case report and case series studies are reported in Table 1. These retrieved studies reported the data of 56 participants overall (40 males [71.42%] with a median age of 60.53 years). These studies were mainly located in Italy28-30,35,38,47 and the USA.39,43,48-50,53
The reported thromboembolic events were PE, venous thromboembolism, arterial thromboembolism, ischaemic stroke, myocardial infarction, and arterial thromboembolism. The most frequent thromboembolic event was PE, which was reported in 15 studies. Comorbidities were evaluated in 27 participants (48.21%). Ischaemic stroke was reported only in the studies of Oxley et al.50 and Moshayedi et al.39 The first study, included five patients admitted with severe large-vessel stroke according to National Institutes of Health Stroke Scale (NIHSS).50 In the second study39 the ischaemic stroke happened after coronary angioplasty and stent deployment, as the patient was admitted for an ST-elevation myocardial infarction. Myocardial injury alone was reported only in two patients,39,42 both in their eighties.
Risk of Bias of the Included Studies
Confounding factors were unclear in observational studies as showed in risk of bias assessment.17,20,23,25 Questions related to confounding factors were not applicable to some observational studies.18,21,22,26-27,54 Adverse events questions did not apply to case reports.
Meta-Analysis Results
Fourteen studies were considered in the meta-analysis. The correlation among event rates is estimable only for mortality and thromboembolic endpoints, reporting more than two pairwise event rates across studies. The correlation matrix among outcomes event rates is higher across PE and VTE outcomes, but it is not significant for all the pairwise comparisons. Some events were rare in the considered study populations; for example, the multi-variable pooled event rate of acute myocardial injuries was estimated to be 0.03 (95% CI: 0.00–0.07; p=0.23), while the meta-analytical estimate of disseminated intravascular disease is 0.04 (95% CI: 0.00–0.08; p=0.03) (Figure 2) . Conversely, other events were found to be more frequent, the pooled proportion of pulmonary embolism was 0.14 (95% CI: 0.08–0.20; p<0.001), while the venous thromboembolism rate is 0.15 (95% CI: 0.09–0.30; p=0.04) (Figure 2). The pooled in-hospital mortality rate was equal to 0.12 (95% CI=0.08–0.16; p<0.001). The multi-variable funnel plot does not reveal publication bias across reported evidence, only the study by Llitjos et al.18 was outside the triangle.
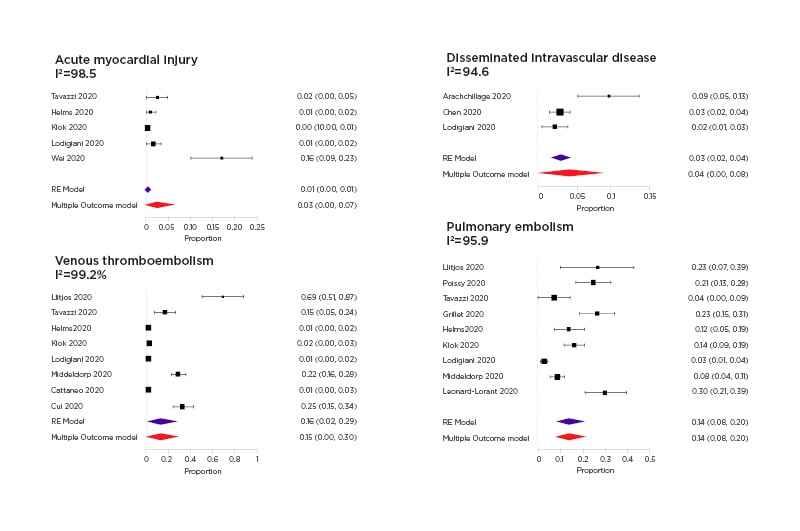
Figure 2: Forest plots of acute myocardial injury disseminated intravascular disease, venous thromboembolism, and pulmonary embolism.
The random effect multi-variate pooled estimate has been reported together with the separate random effect meta-analysis pooled estimate with the 95% confidence interval.
The multi-variable and univariate pooled estimates were similar especially for reported outcomes in more than six studies.
The I2 heterogeneity levels, for all the reported outcomes (AMI, PE, VTE, disseminated intravascular disease, mortality), reveals a substantial heterogeneity with values exceeding the 60%. A significant modifying effect of the country (-0.081; 95% CI: -0.133, -0.029; p=0.0024) and study design (0.061; 95% CI: 0.007, 0.114; p=0.0274) has been evidenced. Moreover, mean age (0.009; 95% CI: -0.012, -0.005; p=<0.001) explains a considerable amount of heterogeneity by bringing I2 values below the 75% threshold as indicated in the literature.55
DISCUSSION
This meta-analysis showed that thromboembolism, particularly PE and VTE are frequent in patients hospitalised for COVID-19 with a pooled hospital mortality rate of 0.12. Coagulopathy is a common complication in patients with severe forms of COVID-19. In a recent study of 191 patients with COVID-19, 50% of in-hospital deaths were in individuals who had coagulopathy, compared to 7% of survivors. Of note, D-dimer levels >1,000 μg/L were associated with death,56 while IL-6 levels may correlate with disease severity, and a procoagulant profile.6,57
In a recent study on 183 patients, International Society on Thrombosis and Haemostasis (ISTH) criteria for disseminated intravascular coagulation (DIC) were met in 71% of patients who died.4 Some studies have reported that the incidence of DIC episodes were lower, ranging from 2% to 9% in patients admitted to ICU. Arachchillage et al.14 reported a higher incidence, probably because their patients had a great percentage of comorbidities 75 (41%) and higher levels of D-dimer (66,000 ng/mL).
SARS-CoV-2 may activate the coagulation system by using different pathogenetic mechanisms such as endothelial dysfunction, systemic inflammation, and a procoagulatory state; this may lead to pulmonary arterial thrombosis.58-60
Moreover, the clinical presentation of PE may overlap with that of COVID-19 pneumonia and may hinder the recognition of PE symptoms in patients who are already complaining of dyspnoea. Grillet et al.17 and Leonard-Lorant et al.25 reported higher proportions of PE compared to other studies, likely because they only included patients who underwent CT angiogram. Poissy et al. compared the prevalence of PE in patients admitted in ICU in the same period of 2019 and 2020. Among patients in ICU with COVID-19 they reported 22 (20.6%) PE cases, which correspond to an absolute increase of 14.4% (95% CI; 6.1–22.8%) against control group of ICU patients admitted from February 27th to March 31st 2019.21
The authors suggest that actual estimates are more likely an underestimation of the real thrombosis prevalence in COVID-19. This has been confirmed by a recent autopsy study54 where deep VTE was found in 58% of the cohort, and 30% of the patients had a PE as the direct cause of death. A recent metanalysis on 3,487 patients shown an incidence of VTE of 26% (95% prediction interval: 6, 66%) with an incidence of PE with or without DVT in 2% of patients (95% prediction interval: 2, 46%).5
Even in the studies where antithrombotic therapy (low-molecular weight heparin at prophylactic or therapeutic dosage) was routinely administered, the risk of thromboembolic complications seemed to remain considerable.
This study confirms that in patients in ICU, the risk of thromboembolism is particularly high; therefore, use of therapeutic dose of anti-thrombotic drugs may be preferable. Despite this, the vast majority of the selected studies involved patients admitted to ICU, the incidence of VTE in patients with COVID-19 appeared to be higher than previously reported in critically ill patients with sepsis or septic shock.61 Llitjos et al.,18 in particular, reported a high prevalence of VTE (69%), which could be attributable to their performing of complex duplex ultrasound on admission as a standard of care in their ICU. In the studies with higher prevalence of VTE, except for Cui S et al.22 and Middeldorp S et al.,20 part of the cohort was sustained by invasive mechanical ventilation.18,26
Regarding myocardial events, the prevalence of myocardial lesions was found to be higher only in the study by Wei et al.27 In fact, they reported an incidence of 33%, which is far above the 0.01% reported by Lodigiani et al.19 and Tavazzi et al.26 This is may be related to the use of troponin levels as the only diagnostic criteria for myocardial injury (14 pg/mL),27 and this may overestimate the rate of true AMI due to embolic events, as differential diagnosis of elevated troponin levels includes nonspecific myocardial injury, impaired renal function, pericarditis, myocarditis, and shock, among others.
Limitations
This is a prevalence metanalysis: the study aim was to analyse prevalence of thromboembolism in patients affected by COVID-19. Predictors of thromboembolism events were not evaluated due to the lack of information about the characteristics of patients in the retrieved articles. For the same reason, the role of thromboembolism in affecting mortality risk was not assessed.
Although the authors chose to exclude case series and case reports from the meta-analysis, the results may have some limitations. The authors are not able to stratify the analysis according to pharmacological profile or characteristics of the patients due to the lack of information on anticoagulant therapy and characteristics of the patients. Further studies are needed to evaluate the impact of different antithrombotic treatments on thromboembolic events in this population. The design of the studies also reduced the quality of the results, as only one study had a form of group control.
CONCLUSIONS
This meta-analysis showed a high prevalence of thromboembolic events in patients with COVID-19, in particular of VTE and PE. These findings may be of great clinical relevance, strongly suggesting to query PE in patients with COVID-19 when haemodynamic deterioration occurs, despite use of prophylactic antithrombotic treatments. These findings encourage the use of therapeutic dose of antithrombotic drugs in patients with COVID-19 in ICU. Further prospective studies are urgently needed to value the effect of prophylactic anticoagulant in such patients.








