Abstract
Ischaemic mitral prolapse (IMP) is a pathologic entity encountered in about one-third of patients undergoing surgery for ischaemic mitral regurgitation. IMP is generally the result of a papillary muscle injury consequent to myocardial infarction, but the recent literature is progressively unveiling a more complex pathogenesis. The mechanisms underlying its development are the impairment of one or more components of the mitral apparatus, which comprises the annulus, chordae tendineae, papillary muscle, and left ventricular wall. IMP is not only a disorder of valvular function but also entails coexistent aspects of a geometric disturbance of the mitral valve configuration and of the left ventricular function and dimension. A correct understanding of all these aspects is crucial to guide and tailor the correct therapeutic strategy to be adopted. Localisation of prolapse and anatomic features of the prolapsed leaflets and the subvalvular apparatus should be carefully evaluated as also constituting the major determinants defining patient outcomes. This review will summarise our current understanding of the pathophysiology of and clinical evidence on IMP, with a particular focus on surgical treatment.
STATE OF THE ART
Ischaemic mitral prolapse (IMP) is a common and easily overlooked valvular dysfunction, secondary to papillary muscle (PM) injury after myocardial infarction (MI).1-6 The prevalence is estimated to be in more than one-third of patients with ischaemic mitral regurgitation (IMR), and there is a male predominance of approximately 3:1.4 The complication of prolapse is due to a regional ventricular injury or wall motion abnormality, rather than a global left ventricular (LV) dysfunction,7,8 and the clinical significance is more daunting than any other ischaemic cardiac lesion.9,10 Despite its importance, our understanding of IMP disease is incomplete and questions remain unanswered about the morphological and functional impairment of the annulus and subvalvular apparatus.11-15 Although much of the original focus is centred on the abnormal restriction of the valve leaflets, the disease is significantly more complex.16,17 IMP is not only a disorder of valvular function but also entails coexistent aspects of a geometric disturbance of the mitral valve configuration and of the LV function and dimension.13,18,19 This review will summarise our current understanding of the pathophysiology, clinical use, and clinical evidence of IMP disease with a focus on surgical treatment.
PATHOPHYSIOLOGY
The mitral valve and subvalvular apparatus encompasses the annulus, valve leaflets, chordae tendineae, PM, and LV wall (Figure 1).
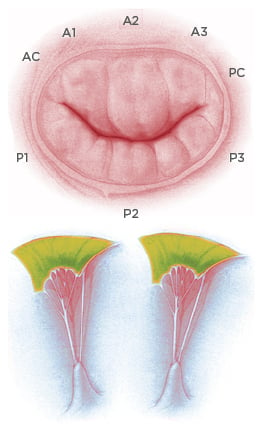
Figure 1: Anatomy of the mitral valve apparatus.
Top: Mitral valve and anatomical description of anterior, posterior leaflet, and relative scallops. Bottom: Representation of papillary muscle and chordae tendineae with attachment on the mitral leaflets.
The valve has anterior and posterior leaflets, and each leaflet typically consists of three distinct segments or scallops; namely, P1, P2, and P3 in the posterior mitral-valve leaflet, and A1, A2, and A3 in the anterior leaflet. The valve leaflets receive chordae tendineae from the anterolateral (AL) and posteromedial PM. Competence of the mitral valve relies on the co-ordinated interaction of the valve and subvalvular apparatus.
Papillary Muscle Morphology
Pioneering work in mitral anatomy showed a range of morphological diversity of PM anatomy and led to an anatomical classification with important implications for IMP surgery.20 Intraoperative inspection of the mitral valve allowed a classification to be compiled, which includes five segmentation and morphological types of the PM:3,4 Type I, single uniform unit; Type II, groove with two apexes; Type III, fenestrations with muscular bridges; Type IV, complete separation in two adjacent heads; and Type V, complete separation with two distant heads. Division can occur according to two directions corresponding to a sagittal plane or to a coronal plane. One leads to a separate posterior leaflet head, whereas the second leads to a separate commissural head. On the basis of this classification, the mechanisms of ischaemic mitral valve prolapse result in: a) necrosis of a separate commissural head inserted close to the annulus, with rupture of the anchorage of the commissural chord; b) necrosis of a single head PM subdivided in multiple heads with partial rupture; or c) necrosis of a fenestrated PM, with detachment of its main insertion favouring an ‘incomplete’ rupture. With time, incomplete rupture mimics PM elongation. In anterior MI, the most common mechanisms of IMP are represented by single PM with complete and total rupture (d) (Figure 2).
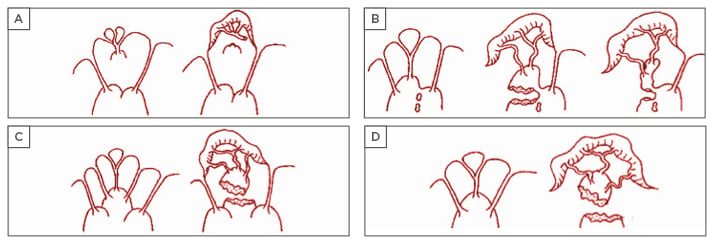
Figure 2: Mechanisms of ischaemic valve prolapse.
From these classifications, it is possible to extrapolate other potential classifications of IMP, including a ‘true prolapse’ (i.e. rupture of PM) and ‘pseudo prolapse’, as in conditions of functional mitral regurgitation (MR) associated to excessive symmetric or asymmetric tethering, but in the absence of clear evidence of PM rupture (myocardial ischaemia with LV remodelling and/or PM dyssynchrony). Also, from the clinical point of view, an ‘acute’ presentation (i.e. acute PM rupture) might be differentiated from a ‘chronic’ presentation in cases of chordal elongation or chronic partial PM rupture. This clinical classification is important for guidance in surgical timing and strategy, because the majority of reparative techniques, such as subvalvular apparatus surgery, are indicated in more chronic circumstances.
Distribution of Coronary Blood Flow in Papillary Muscles
The uneven coronary distribution and the differences in PM anatomy account for a heterogeneous morphological variability of papillary damage. Asymmetric distribution of blood supply accounts for the rare involvement of the anterior PM, which is perfused by both the left anterior descending coronary artery and diagonal branch. In addition, the tension exerted by the chordae on this PM is relatively low due to its superficial location with regard to the annulus. Conversely, the posterior PM is more sensitive to ischaemia (91% of the cases in our series),3,21 because its blood supply relies on distal branches exclusively furnished by either the right coronary or the circumflex artery; furthermore, its location deep in the LV subjects this muscle to a higher shear force. PM microcirculation relies on both an independent blood supply, provided by a well-identified arterial trunk perforating the PM from base to apex (Kugel’s artery), and a segmental distribution.20 However, features of microcirculation imbricate with anatomical characteristics of PM, and the relative importance of one of the two circulatory systems depends on the morphology and position of the PM within the ventricle and on the presence of muscular bridging, which favours collateralisation. Clearly, the importance of the truncal system increases as the PM is more individualised from the ventricular wall, as in Type IV–V, with the apex becoming more prone to rupture due to the fragility of its truncal blood supply and to the degree of physical stress. Partial PM rupture or elongation limited to a single head are therefore more likely to occur than in cases of multiple muscular bridges that guarantee an adequate collateral compensation.
The morphology of the posterior PM, which is the usual site of ischaemic injury, is more complex than the anterior PM, and its subdivision into several heads is very frequent. IMP is frequently caused by a partial PM rupture or elongation limited to a single head. Alternatively, prolapse can be favoured by an incomplete detachment of a head due to a rupture of its main insertion with the body while remaining fixed to the ventricle via muscular bridges (‘incomplete’ PM rupture). The incidence of IMP is typically unequal for topographic distribution in the leaflet. In the majority of patients, the disease is manifested by a prolapse of the posteromedial commissure, extended often in A2-A3 scallops.2-4,22 The PM commissure and the neighbouring scallops functional Type II Carpentier classification.23 This anatomic abnormality results in the mitral orifice not closing completely during systole, causing regurgitation.24 The IMP involves an imbalance between tethering forces and closing forces in the valvular and subvalvular apparatus.2,22,25-30 Accumulating evidence suggests that the presence of primary lesion or dysfunction of PM leads to prolapse in 86.4% of IMR patients.21,31 In our series, ALPM was involved in 18.2% and posteromedial PM in 63.8%. In 13.6%, the prolapse was determined by necrosis of a restricted area of the myocardium adjacent to the PM, which was responsible for its abnormal traction and its dyssyncrony.4
CLINICAL EVIDENCE
Although the clinical presentation of patients with IMP can vary from severe to moderate valve regurgitation, evidence from observational series strongly suggests that surgical intervention is beneficial.1,32-35 We evaluated the effect of early surgery on long-term outcomes in 75 patients with mitral regurgitation for IMP. Patients with total rupture of PM and cardiogenic shock showed a higher rate of death in comparison to patients in which the culprit morphological injury was chordae lesion or incomplete rupture.3,4,36 The clinical manifestations were related to the residual function of the mitral valve (severe or moderate incompetence), extension of MI, residual myocardial ischaemia, and acquired complications, such as LV dysfunction and rhythm disturbance.1,36
Also, there is evidence that suggests that the presence of a mitral leaflet prolapse in IMR should represent an indication for surgery of the mitral valve in combination with coronary artery bypass grafting.35 Earlier studies on non-corrected mitral regurgitation in the context of IMP date back to the era of cardiac catheterisation.37-39 The late outcomes of patients with coronary disease and moderate mitral valve dysfunction with leaflet prolapse who have not undergone mitral repair have been studied.39,40 Although results are not univocal, estimates of the prevalence of complications and outcomes have varied depending on the era of the study, the cohort selected, and the method used to diagnose IMP (clinical exam versus cardiac catheterisation versus echocardiography). To our knowledge, two large recent series have helped to better define the clinical course of unoperated IMP in the modern era.37,41-43
CLINICAL USE
Patients with IMP should have a careful assessment of symptoms after MI and should undergo electrocardiography, primarily to evaluate localisation and extension of the necrosis and secondly to evaluate cardiac rhythm. Transthoracic echocardiography should always be performed to assess the mechanism and severity of IMP, as well as LV size and function. A semiquantitative scale is often used to grade mitral regurgitation: 1+ (trace), 2+ (mild), 3+ (moderate), and 4+ (severe).44 Patients with a total rupture of ALPM who have severe mitral regurgitation with cardiogenic shock symptoms, pulmonary oedema, severe LV dysfunction (ejection fraction, <40%), or dilatation (LV end-systolic dimension, >40 mm) should be presented quickly to a surgeon.45 Likewise, symptomatic patients with moderate LV dysfunction or dilatation, with or without atrial fibrillation or pulmonary hypertension, should be considered for surgery after 60 days.3,4 In not extended inferior MI, patients with chordae lesion injury, mild-to-moderate mitral regurgitation, and no evidence of LV dysfunction or dilatation should be observed until the development of either symptoms or severe mitral regurgitation. Frequently, these patients have a percutaneous coronary intervention-stenting procedure.3,4
Before the advent of mitral valve repair, valve replacement was the preferred procedure for severe IMR alone or with leaflet prolapse.22 Valve replacement may still be preferred in certain situations, such as in patients with advanced age, total PM rupture, and severe LV dysfunction, in which a combined or complex surgical procedure is needed.45 In the case of extensive prolapse of A2-A3 scallops, mitral valve sparing operations should be the preferred option.32 In such cases, chordae sparing valve replacement for MR may be a suitable alternative to repair the leaflet prolapse. Individual and institutional experience is crucial in determining the likelihood of a repair procedure’s success. Many centres worldwide report the lowest mortality rates with the highest proportion of patients undergoing mitral valve repair rather than replacement.46 Current debate on IMR is animated by several studies introducing parameters or variables predicting the prognosis or outcome of mitral repair. Kron et al.47 reported a set of clinical and echocardiographic variables able to predict recurrence of MR after surgical repair.1,2,28,48 On account of these results, the surgeon should precisely evaluate the likelihood of successful repair, in light of his or her own experience, when counselling the patient and may recommend a second opinion. Failure of the repair can lead to conversion in mitral replacement; the decision between a mechanical valve and a bioprosthesis should be discussed with the patient before the operation. After IMP surgery, a functional Type II residual MR with a structurally normal mitral valve may occur. In addition, cases of systolic restricted leaflet motion on the remaining leaflets (Type III-b) and some degree of annular dilatation (functional Type I) may also be present, highlighting the aforementioned imbalance between tethering forces and closing forces in IMP.23,24
In our group, we have treated 214 patients with surgery for IMR and 75 of those had IMP. Mitral valve repair was performed in 90.7% of cases and valve replacement was required in 9.3% of cases.3,4 Intraoperative observation revealed leaflet prolapse with structurally normal mitral valve (functional Type II) in all patients. The mechanism of prolapse was PM injury in 88% of cases and chordal injury in 12% of patients. Anatomical classification of PM injury demonstrated an ALPM lesion in 20% of the patients, posteromedial PM lesion in 66.7% of cases, and posteromedial PM elongation in 13.3% of the population considered. Patients with isolated total PM rupture, or a partial rupture with an extensive prolapse of A3-PC-P3 scallops, had a mitral valve replacement. The posteromedial commissure prolapse, which was encountered in the majority of the patients, was repaired with isolated stitch. Various techniques may be used to repair the anterior leaflet, including artificial chordal replacement with Gore-Tex® (expanded polytetrafluoroethylene sutures), chordal transposition, and limited triangular resection. The prolapse of the posterior middle scallop (P2), which is encountered in a minority of patients with IMR, is usually repaired with limited resection of this scallop. Finally, in all cases, a downsized annuloplasty was performed to stabilise the annulus, which was normally found to be distorted, dilated, or both.49
Another important point thoroughly discussed in the literature are the real benefits of PM treatment in reducing LV dysfunction in IMR surgery. Different types of procedure are codified in this context: PM approximation, PM sling, and PM relocation.50,51 Several procedures have been compared, with PM approximation being an effective alternative among the available options52 (Figure 3), but there are no large randomised trials in which the real benefit of those procedures in the long-term follow-up is demonstrated. In this context, a recent propensity-matched study demonstrated that the combination of ring annuloplasty and PM sling produced a significant improvement in mitral valve apparatus geometry and resulted in reduction of MR recurrence in the early postoperative period.53 Similarly, a randomised control trial and its further sub-analysis demonstrated the superiority of combined restrictive annuloplasty and PM approximation over the simple annuloplasty, in terms of LV remodelling and cardiac outcomes.54,55 A recent meta-analysis summarised the current evidence on this argument and demonstrated that combined subvalvular procedures plus mitral annuloplasty are associated with greater LV reverse remodelling and systolic function, less recurrence of moderate or greater MR, and an improved geometry of the MV apparatus at short and mid-term follow-up.56
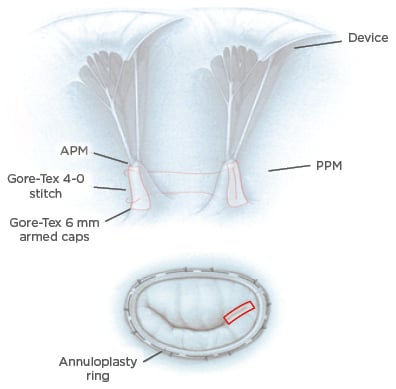
Figure 3: Surgical reparative strategies.
Top: PMA. A Gore-Tex® cap is used to reinforce the heads of the papillary muscle and a Gore-Tex 4-0 suture is used to approximate the two papillary muscles and therefore increase leaflets coaptation. Bottom: mitral annuloplasty performed in association with PMA (red rectangle).
APM: anterior papillary muscle; PMA: papillary muscle approximation; PPM: posterior papillary muscle.
In our series, concomitant coronary artery bypass grafting was accomplished in all patients using an internal thoracic artery with left anterior descending artery lesion. We routinely advise intraoperative transoesophageal echocardiography during IMP surgery to evaluate the effectiveness of the repair. Transoesophageal echocardiography provides precise anatomic and functional data that are helpful in understanding the mechanism and severity of mitral regurgitation, including the extent of leaflet prolapse, the condition of the subvalvular apparatus, the diameter of the mitral annulus, and ventricular function, and are therefore fundamental in planning the best operative strategy.44 We recommend a postoperative echocardiographic follow-up after mitral valve repair 6–8 weeks after discharge. Usually, patients are then transferred to the care of their cardiologist and family physician, and we advise the echocardiography be performed annually thereafter.
COMPLICATIONS AND PITFALLS
Mitral valve repair or replacement in IMR alone or with leaflet prolapse is associated with an operative mortality of ≤6%. In our experience, the most frequent primary causes of death were found to be multisystem organ failure (37.5%), heart failure (12.5%), and renal failure (10%). Predictors of death include advanced age and poorer New York Heart Association (NYHA) class. This condition is frequently encountered in patients who underwent hospitalisation for PM rupture and cardiogenic shock associated with extended anterior MI.41 Other determinants are represented by lower preoperative ejection fraction, high preoperative LV end-systolic dimension, and other coexisting conditions, including diabetes, renal disease, chronic lung disease, and obesity. In an analysis from the Society of Thoracic Surgeons (STS) National Adult Cardiac Surgery Database, the major postoperative complications before discharge included prolonged (>24 hours) ventilatory support (10.4% of patients), renal failure (4.8%), and stroke (2.4%). Thromboembolism after mitral valve repair occurred in approximately 2.8% of patients within the first 12 months after surgery. Intraoperative conversion to mitral valve replacement occurred in 2–10% of cases.36,45 The most important complication of mitral valve repair is recurrent MR, which may occur in as many as 5–30% of patients requiring reoperation.47,57
Considering our series estimates of IMP, the rate of late cardiac events (medical and surgical complications) were approximately 34.7%, with a mean survival of 114.2 months, including patients with in-hospital mortality. After 5 and 10 years, survival from cardiac-related events was 75% and 50%, respectively. Cardiac event rates were higher if one or more risk factors were present: age >70 years, total rupture of ALPM in anterior MI, severe mitral incompetence, cardiogenic shock, and surgery before 30 days. In our cohort, 76.5% of patients with IMP had no significant MR after surgery within 10 years of follow-up, while 24% of the patients required reoperation. Of note, the majority of patients developed rhythm disturbances before operation, namely atrioventricular blocks and QRS prolongation, suggesting that the appearance of these abnormalities should prompt decision towards some form of intervention. However, further investigations are warranted to confirm this finding.
CONCLUSION
IMP is a daunting condition in IMR, which deserves full consideration in terms of both diagnosis and treatment. The presence of IMP is regarded as an indication to perform surgery, as it underlies more than a simple annular dilation, which can be mainly addressed by myocardial revascularisation and interrupting the ventricular remodelling process. Conversely, the appearance of IMP indicates an alteration of more than one of the components of the mitral apparatus and valve configuration (annulus, PM, chordae, LV geometry) and therefore requires more careful attention in the operative work-up. In this scenario, PM surgery is still a debated argument and there is no final answer on the correct surgical approach to use. A randomised trial to elucidate this point is needed in this context.
FUTURE DIRECTIONS
IMP is a challenging disease and a comprehensive understanding is necessary in order to correctly address its repair. Thanks to improvement in imaging techniques, more and more sophisticated analyses are achievable, with the possibility to combine them with advanced mathematical modelling of the mitral valve. Approaches such as finite element analysis (FEA) enable us to precisely dissect the pathogenic events occurring in IMP to evaluate the changes induced by the application of different operative techniques.58-60 In the future, application of these and other in silico studies at the preoperative stage may be extremely helpful to plan the optimal strategy to be adopted for each patient. However, to produce a clinically usable patient-specific algorithm, more data and subgroup analyses are still required.








