Abstract
Cardiac disease is an important cause of mortality in pregnancy. It has the potential to remain undiagnosed and may present with cardiovascular decompensation during pregnancy, at the time of delivery, or immediately postpartum. It can have long-term implications to the life of the affected women and their families. This review summarises the current knowledge of the incidence, prevalence, and management of pregnancy-related cardiovascular disease in women presenting preconceptionally or during pregnancy.
INTRODUCTION
Cardiovascular diseases complicate approximately 0.2–4.0% of pregnancies.1 Their incidence is increasing, and they are already the most common cause of maternal death in the UK.2 These patients are at higher risk of mortality as well as increased morbidity and thus need special care during the period of pregnancy.
PREGNANCY CHANGES IN MAJOR BODY ORGAN-SYSTEMS
Several changes during pregnancy in a normal patient can occur:
- Central nervous system changes: decreases in minimum alveolar concentration3 and local anaesthetic requirement during neuraxial blocks.4
- Respiratory system changes: development of upper airway oedema, decreases in functional residual capacity, and increases in ventilation.5
- Cardiovascular system changes: increases in blood volume, cardiac output (CO), and development of supine hypotension syndrome.6,7
- Gastrointestinal system changes: altered gastric emptying, increased gastrin secretion, and increased likelihood of gastroesophageal reflux.8
- Renal and hepatic changes: increased glomerular filtration rate and raised levels of liver enzymes.9
These changes are usually well-tolerated in normal patients; however, in patients with pre-existing disorders of body systems these changes can cause acute decompensation of disease status and can be catastrophic.
In this review the management of pregnant patients with cardiac disease is discussed, beginning with the discussion of the cardiovascular system changes in normal pregnancy followed by management of specific cardiac diseases during pregnancy.
LITERATURE SEARCH AND SELECTION
For the literature search, an electronic search in Google Scholar, PubMed, and Cochrane Databases for original and review articles on cardiac disease in pregnancy until March 2019 was performed. Only full text articles were included.
CARDIOVASCULAR CHANGES IN NORMAL PREGNANCY
The changes in the cardiovascular system evolve as a pregnancy advances.
Changes in the Left Ventricle
The left ventricular (LV) mass increases during pregnancy.10 The LV diastolic function increases during the first two trimesters and falls in the third trimester.10 Pre-eclampsia patients and patients with multiple gestation show a greater increase in LV mass.11,12
Changes in Cardiac Output
Increased levels of the hormone progesterone cause peripheral vasodilation leading to a decrease in systemic vascular resistance. As a response to decreased systemic vascular resistance, CO increases progressively as the duration of pregnancy increases. Although an increase in both heart rate and stroke volume contribute to increased CO, the increase is predominantly a result of augmented stroke volume.6 By 8 weeks, CO has increased by 20% and then up to 50% by 20 weeks.13 The CO further increases by 15% during the first stage of labour.14,15 After delivery of the baby, autotransfusion of approximately 300–500 mL of blood from uterine circulation to maternal circulation occurs. This can lead to an increase in CO of up to 60–80% during the second stage of labour.16 In case of twin pregnancies, there is an additional 10-20% increase in CO.17
Changes in Blood Pressure
Systolic, diastolic, and mean blood pressure decrease during mid-pregnancy and return toward baseline as the pregnancy approaches term.18 The mean (±2 standard deviations) systolic blood pressure and diastolic blood pressure in women who have never given birth at 12 weeks have been described to be 112.1 mmHg (88.6–135.5 mmHg) and 65.4 mmHg (48.9–81.9 mmHg), respectively.19 It has been suggested that a raised mid-trimester mean arterial BP is predictive of subsequent development of pregnancy-induced hypertension/pre-eclampsia.20
Total Blood Volume Changes
Pregnancy is also characterised by an increase in total blood volume. This increase begins as early as 6 weeks of pregnancy and is rapid during the first half of pregnancy, after which the increase in blood volume progresses at a slower rate.21,22 There is also an increase in red blood cell mass during pregnancy; however, this increase is to a lesser extent compared with the increase in blood volume. Hence, there is haemodilution and resultant development of ‘physiological anaemia of pregnancy’.
ECG Changes
ECG changes during pregnancy consist of nonspecific ST-segment and T-wave changes. Repolarisation abnormalities are absent in normal pregnancies. LV mass increases during normal pregnancy.10
ASSESSMENT OF PREGNANCY RISK
All female cardiac patients should undergo a preconception counselling, which should include a detailed discussion of the risk of pregnancy. There are numerous important issues to be considered during this counselling:
- Evaluation of present cardiac status using history, clinical exam, and relevant investigations.
- Optimisation of cardiac status.
- Changes in medication regimen, e.g., replacing teratogenic drugs with nonteratogenic drugs.
- Discussion about maternal life expectancy and long-term effects of pregnancy on their heart.
- Genetic screening in patients with inherited disorders.
CARPREG RISK SCORE
Siu et al.23 had proposed the Cardiac Disease in Pregnancy (CARPREG) Risk Score to estimate a woman’s cardiac risk during pregnancy. During the calculation of the total score, one point is assigned for each of the four risk factors:
- A history of cardiac event or arrhythmia,
- New York Heart Association (NYHA) functional class greater than II or cyanosis,
- left-heart obstruction (mitral valve area <2.0 cm2, aortic valve area <1.5 cm2, or LV outflow tract gradient >30.0 mmHg), and
- LV ejection fraction (LVEF) <0.40.
A score of 0 points confers a 5% risk of cardiac complications, whereas scores of 1 or 2 points denote a 27% and 75% risk, respectively. Post CARPREG, many such risk stratification models have been suggested by other bodies, prominent among them being World Health Organization (WHO) classification, ZAHARA risk score, European Society of Cardiology (ESC) guidelines, plus more. To determine the risk of pregnancy in cardiac patients, WHO has classified patients into four pregnancy risk classes (Classes I–IV) as determined by their medical condition (Box 1).
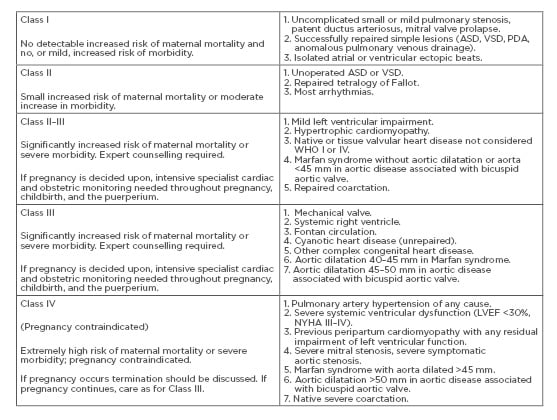
Box 1: Modified World Health Organization (WHO) classification of maternal cardiovascular risk.
ASD: atrial septal defect; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PDA: patent ductus arteriosus; VSD: ventricular septal defect; WHO: World Health Organization.
MANAGEMENT OF CONGENITAL HEART DISEASES
Atrial Septal Defect
Patients with unrepaired atrial septal defect (ASD) should have their right ventricular function assessed. It has been suggested that pregnant women with an ASD are more likely to develop supraventricular and ventricular arrhythmias than nonpregnant women.24 In patients with unrepaired ASD the risk of paradoxical air embolism is present. Patients with repaired ASD can be managed as normal patients; however, ASD (both repaired or unrepaired) patients with pulmonary arterial pressure >40 mmHg are considered as high-risk patients and preload should be maintained.
Ventricular Septal Defects
Pregnancy is usually well-tolerated in patients with ventricular septal defects. The only risk from a small haemodynamically insignificant ventricular septal defect is of endocarditis, and antibiotics should be administered at the time of instrumental or complicated deliveries. However, patients with raised pulmonary arterial pressures (>40 mmHg) are considered high risk. Additionally, women with either unoperated ventricular septal defects or with late repair may have associated pulmonary vascular disease.
Patent Ductus Arteriosus
Usually pregnancy is well-tolerated in patent ductus arteriosus patients with complications being rare.25 However, in patients who have developed reversal of shunt (Eisenmenger syndrome), pregnancy is not recommended because of the risk of death reaching as high as 40–50%.26
Transposition of the Great Arteries
There are two types of transposition of the great arteries (TGA): D-TGA, in which only origins of aorta and pulmonary trunk are transposed; and L-TGA, in which the morphological left and right ventricles with their corresponding atrioventricular valves are also transposed, in addition to aorta and pulmonary trunk. D-TGA patients undergo either arterial (Jatene or Rastelli) or atrial switch (Senning or Mustard) operations. The patients who have undergone arterial switch operation are predisposed to develop myocardial ischaemia (because the coronary arteries are reimplanted during these procedures), while patients with atrial switch can be afflicted by pulmonary hypertension, atrial arrhythmias (as a result of atrial scarring), tricuspid regurgitation, plus more.27 In these patients, a cardiac evaluation and ECG/MRI are recommended prior to planned pregnancy. Pregnancy is usually well-tolerated in NYHA Class I–II patients post-Mustard procedure.28 Patients with L-TGA usually have uneventful pregnancies.
Ebstein’s Anomaly
Ebstein’s anomaly is a congenital heart defect in which the septal and posterior leaflets of the tricuspid valve are displaced towards the apex of the right ventricle of the heart.
Patients with preserved ventricular function tolerate pregnancy well, whereas those with associated ASD and cyanosis have an increased risk for fetal loss.29 Additionally, arrhythmias are very common in these patients. Severe Ebstein’s anomaly should be repaired prior to pregnancy.
Tetralogy of Fallot
It is rare to have tetralogy of Fallot patients survive to adulthood without corrective surgery. Women with repaired tetralogy of Fallot and well compensated haemodynamic function tolerate pregnancy well, though they remain at risk for atrial and ventricular arrhythmias. However, the presence of pulmonary hypertension, right ventricular dysfunction, right ventricular dilation, and pulmonic regurgitation predisposes these patients to adverse peripartum complications such as arrhythmias and right-sided heart failure.
Fontan Circulation
In Fontan surgery, the right ventricle is bypassed and venous blood flows directly from vena cavae to pulmonary arteries. Fontan operation is performed for tricuspid or pulmonic atresia, as well as other anomalies with a single ventricle. These patients are predisposed to the development of thrombus formation (due to slow flow of venous blood) and arrhythmias (as a result of surgical scar tissue in the atrium).30,31
Management of Acute Coronary Syndrome
Acute coronary syndrome (ACS) includes ST elevation myocardial infarction (STEMI), non-STEMI, and unstable angina. ACS during pregnancy is rare, with an incidence of 1 in 16,000 deliveries.32 The classical presentation of ACS presenting as crushing chest pain with radiation to neck and arm is uncommon, and even in those patients who present with these classical features, often these symptoms are attributed to dyspepsia and changes of normal pregnancy. Hence diagnosis may be difficult.
STEMI should be a clinical diagnosis based on the presence of pain with typical ECG changes (either a 1 mm of ST elevation in contiguous leads corresponding to an arterial territory or new left bundle branch block). All patients with a history of pain that may be a result of cardiac ischaemia should have a prompt 12-lead ECG.
Diagnosing non-STEMI will be based on the presence of elevated cardiac troponin levels (or a documented rise and fall) in the setting of pain compatible with cardiac ischaemia. Unstable angina may present similarly to non-STEMI but without an elevated troponin level. Often these patients are still at high risk of future events or development of more extensive ACS if not managed appropriately.
In patients who are presenting with stable symptoms (symptoms or exertion), noninvasive investigations of cardiac ischaemia are the preferred management. Exercise testing is safe in pregnancy provided the patient is not having obstetric complications such as per vaginal bleeding or significant placenta praevia. The disadvantage of this test, however, is the high false-positive rate in the nonpregnant woman.
In patients with STEMI
- Oxygen supplementation.
- Aspirin (300 mg) and clopidogrel (300 mg).
- Appropriate intervention in the form of coronary angiography, emergency coronary intervention, and thrombolysis should not be withheld in the pregnant or puerperal woman.33 The first choice for treatment of ACS in pregnant women is percutaneous coronary intervention (balloon angioplasty with or without a stent).34
In patients with Non-STEMI/Unstable Angina
Low-risk patients should be managed medically (aspirin, clopidogrel, low-molecular-weight heparin, other antianginal agents), while high-risk patients should preferably undergo angiography and, if needed, coronary stenting.
MANAGEMENT OF VALVULAR HEART DISEASES
Mitral Stenosis
Even patients with mild mitral stenosis can develop arrythmias and pulmonary oedema during pregnancy. When possible, preconception treatment of symptomatic moderate or severe mitral stenosis is preferred, percutaneous balloon mitral valvuloplasty being the procedure of choice.35 For patients who require percutaneous valvuloplasty during pregnancy, the procedure is ideally performed after 12–14 weeks gestation in order to minimise fetal radiation exposure during the period of organogenesis. If the patient can be stabilised with medical management, delaying the procedure to 26–30 weeks gestation will help reduce the risk for preterm birth. Open surgical mitral valve commissurotomy is another treatment option but is associated with higher rates of fetal mortality than percutaneous valvuloplasty (38% versus 5%, respectively).
Medical management includes administration of β-blockers, aimed at slowing the heart rate and thereby lengthening the diastolic filling period. Atrial fibrillation and atrial flutter should be treated promptly with rate control, and early cardioversion should be considered. Furthermore, systemic anticoagulation is recommended for the duration of pregnancy and postpartum along with diuretics and bed rest.
Mitral Regurgitation
Mitral regurgitation is usually well-tolerated during pregnancy, but ECG is recommended because chronic mitral regurgitation may be associated with LV dysfunction. If valve intervention is indicated in women of childbearing age who have severe mitral regurgitation, valve repair should be offered when possible.
Aortic Stenosis
Mild and moderate aortic stenosis are associated with favourable pregnancy outcomes.36,37 Severe aortic stenosis patients are more predisposed to develop cardiac complications as well as the need for cardiac intervention. Balloon valvuloplasty is preferred if technically possible because it is reported to have a smaller risk of fetal loss, and even if the benefit is short lasting, it may be sufficient to allow successful completion of the pregnancy.
Aortic Regurgitation
Chronic, moderate, or even severe aortic regurgitation is usually well-tolerated if LV function is preserved; nevertheless, women with severe aortic regurgitation are at a risk of developing pulmonary oedema and arrhythmias during pregnancy. Valve replacement during pregnancy for treatment of aortic regurgitation is rarely required.
Pregnant Patients with Prosthetic Valves
These patients have increased chances of morbidity and mortality.38 In the subset of 134 women with bioprosthetic valves in the Registry of Pregnancy and Cardiac Disease (ROPAC) study, heart failure complicated 8.2% of pregnancies in women with bioprosthetic valves, endocarditis and thrombotic complications in <1.0%, and haemorrhagic complications in 5.1% of pregnancies.38 Prosthetic heart valves can either be bioprosthetic or mechanical; the former are often recommended in young females because of a lower risk of thromboembolism and anticoagulation. However, bioprosthetic valves tend to deteriorate early, predisposing to repeated surgeries.
In patients with mechanical prosthetic heart valves, the current American Heart Association (AHA)/American College of Cardiology (ACC) guidelines suggest continuing warfarin in the first trimester if the daily warfarin dose is ≤5 mg, after patient information and consent. In women whose daily warfarin dose is >5 mg, and for those who consent against taking warfarin in the first trimester, it may be discontinued between Weeks 6 and 12 and replaced with either weight-adjusted twice-daily low-molecular-weight heparin or an intravenous infusion of unfractionated heparin. Warfarin is restarted along with aspirin 75 mg in the second and third trimester until 36 weeks of gestation. Overall, pregnancy in women with mechanical prosthetic heart valves is high risk, and the safest option is not to become pregnant at all.
Infective Endocarditis in Pregnancy
Infective endocarditis is a rare, potentially life-threatening complication. Maternal and fetal mortality rates are both high.39 The AHA guidelines on the prevention of endocarditis and the ESC guidelines on the management of cardiovascular diseases during pregnancy do not recommend antibiotic prophylaxis at the time of delivery. If infective endocarditis is diagnosed, antibiotic treatment should be guided by blood culture results and antibiotic sensitivities.
Cardiomyopathy
Cardiomyopathy is a congenital or acquired disease of heart muscle. Congenital cardiomyopathy can be inherited in an autosomal dominant, autosomal recessive, X-linked dominant, X-linked recessive, or mitochondrial manner. Various types of cardiomyopathies exist:
Hypertrophic Cardiomyopathy
In hypertrophic cardiomyopathy, abnormal thickening of heart muscles occurs, rendering the heart muscle stiff and noncompliant. These patients are unable to sufficiently increase their stroke volume as a result of outflow obstruction in conjunction with the small stroke volume due to a small LV cavity size. Patients may be asymptomatic or present with palpitations, breathlessness, or arrhythmias.
Patients with hypertrophic cardiomyopathy are at risk from atrial and ventricular arrhythmia, pulmonary oedema, and increasing outflow tract obstruction. Atrial arrhythmia, especially atrial fibrillation from left atrial dilatation, is common and can cause thromboembolism. Normally, atria contribute around 10–30% of ventricular filling, but in hypertrophic cardiomyopathy patients it may increase to 50% of total ventricular filling. Hence restoring sinus rhythm promptly is imperative.
Dilated Cardiomyopathy
Dilated cardiomyopathy can be primary or secondary to myocarditis, alcohol or other toxins, endocrine and autoimmune disorders, and nutritional factors. Breathlessness, fatigue, exercise intolerance, and fluid retention are common symptoms. Pregnancy in women with dilated cardiomyopathy is associated with adverse outcomes, especially in those with significantly impaired LV function (moderate or severe LV systolic dysfunction, EF <45% on ECG). Assessment of LV function and exercise tolerance is important in these patients preconceptionally. If the patient is being treated with heart failure medication known to have teratogenic effects, such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, they should be stopped prior to conception. If LV function then deteriorates even before pregnancy, the patient should be advised against pregnancy. Thereafter also, ECG should be performed at regular intervals to determine if LV function declines as a consequence of their withdrawal. β-blockers should be continued during pregnancy and postpartum. Other drugs used are diuretics (to manage fluid status) and nitrates or hydralazine (to reduce preload). Pregnancy may have to be terminated at any duration of gestation if LV function deteriorates.
Peripartum Cardiomyopathy
The Working Group on Peripartum Cardiomyopathy (PPCM) of the ESC recently updated the operational definition of PPCM, defining PPCM as cardiomyopathy with reduced EF, usually <45%, presenting toward the end of pregnancy or in the months after delivery in a woman without previously known structural heart disease.40 PPCM is associated with significant morbidity and mortality.41 The exact aetiology of PPCM remains unknown; however, fetal microchimerism, increased cardiomyocyte apoptosis, hormonal insults, genetics, autoimmune inflammation and myocarditis with and without viral triggers, and a familial association have been proposed.42 Most cases present in the first week following delivery.43 Increasing age, multiple gestations, race, and pre-eclampsia have been found to be associated with development of PPCM.44 ECG usually shows dilatation of LV, LV systolic dysfunction, right ventricular and biatrial enlargement, mitral and tricuspid regurgitation, and pulmonary hypertension.44,45
Digitalis is used to augment systolic function and is safe, although its role in the management of systolic heart failure has been questioned.46 Anticoagulation may be added and continued postpartum if the LVEF remains <30% for prevention of thromboembolic complications. Angiotensin-converting enzyme inhibitors should be started as soon as possible after delivery, and are safe for breastfeeding. Bromocriptine, a dopamine receptor agonist and prolactin inhibitor, has showed improvements in LV recovery.47 Cardiac assist devices and implantable cardiac defibrillators have been used in patients with severe depression of LV function and persistent arrhythmias.48,49
Restrictive Cardiomyopathy
Restrictive cardiomyopathy can affect both sides of the heart and is characterised by small, stiff ventricles with abnormal relaxation. As a result, diastolic filling is impaired, which in turn causes small stroke volumes and a low CO, despite an often preserved systolic function.
Pulmonary Hypertension
Pulmonary hypertension (PAH) is defined as an increase in mean pulmonary arterial pressure ≥25 mmHg at rest.50 In patients with congenital heart disease, PAH most commonly occurs as a result of long-term left-to-right shunting, leading to increased pulmonary flow that eventually causes high PVR, resulting in reversed or bidirectional shunts, which is referred to as Eisenmenger syndrome.
Women with PAH should be strongly counselled against pregnancy at the time the diagnosis of PAH, and advice on appropriate contraception should be provided. Pulmonary vasodilators, such as iloprost, inhaled nitric oxide, endothelin receptor antagonists, and phosphodiesterase inhibitors, have been used in these patients and have improved outcomes to some extent.51
Aortic Dissection
Aortic dissection is a particular risk in women with Marfan syndrome. β-blockers should be continued or started in pregnant patients with Marfan syndrome who have aortic dilatation or hypertension because they have reduce the rate of aortic dilatation.52 Monitoring during pregnancy will normally include regular (e.g., every 4–8 weeks) transthoracic ECG to assess aortic root diameter. The timing of delivery will be dependent on the root diameter and the rate of dilatation, as well as any other complicating factors.
COMMON ARRYTHMIAS DURING PREGNANCY
Cardiovascular changes during pregnancy predispose the development of new onset arrhythmias as well as recurrence in patients with a history of arrhythmias.53 Ectopic beats are said to be common during pregnancy and usually do not require any treatment.54
Patients without any history of heart disease usually present with atrioventricular nodal reentrant tachycardia.55 Direct current cardioversion has been found to be safe in all stages of pregnancy; however, fetal monitoring is advised during cardioversion.56 In haemodynamically stable patients, intravenous adenosine is commonly used for termination of atrioventricular node dependent supraventricular tachycardia. Catheter ablation therapy is generally contraindicated due to high radiation exposure. Patients presenting with symptomatic bradycardia and a heart rate <50 beats per minute are candidates for permanent pacemaker insertion.57
RADIATION EXPOSURE DURING PREGNANCY
The effects of in utero radiation exposure include intrauterine growth retardation, childhood cancers, mental retardation, and fetal death. The gestational age, radiation dose, and repair mechanisms determine the final effect of the radiation on the fetus. A dose <0.05 Gy does not cause any malignancy related health issues.58
CONCLUSION
The spectrum of cardiac disease has changed over time, with rheumatic disease becoming uncommon while congenital heart disease becomes more common. As newer and better treatment modalities for the management of cardiac diseases emerge, the number of cardiac disease patients becoming pregnant also continues to increase.
Pregnancy may predispose cardiac disease patients to many complications, some of which may be life threatening. While the risk may be so high in certain disease states that pregnancy should be altogether avoided, successful outcomes are possible in other cases provided close co-ordination and collaboration is maintained between all the members of the treating team.








